Yesterday Actinogen (AX:ACW) announced the initial results from its Phase II clinical trial of Xanamem in patients with mild dementia due to Alzheimer’s disease (AD). The safety of the drug was confirmed and the data showed Xanamem was effectively inhibiting cortisol production, as demonstrated by the expected increase in adrenocorticotropic hormone. However, the 10mg dose of Xanamem was not effective in demonstrating statistical significance on any of the endpoints. We note that both primary and secondary endpoints were robust ‘gold standard’ psychiatric tests used to measure cognition in AD patients, hence the trial was well designed, but the hurdle was high. Actinogen indicated it will analyse the data and make a decision on future steps once that is complete and the results from other supporting trials are released. Specifically, the company indicated that a higher dose and longer treatment could potentially be a way forward, given the drug appears safe and pharmacologically active.
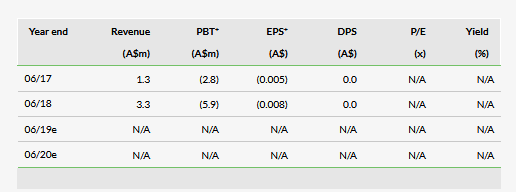
Xanamem is a selective 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1) inhibitor that can cross the blood-brain barrier and target excess brain cortisol, which has been associated with cognitive impairment in AD (described in detail in our initiation report). The Phase II XanADu trial started in March 2017. It was a double-blind, placebo-controlled Phase II trial with mild AD patients (n=186), who received 10mg of Xanamem daily or placebo (1:1) for 12 weeks. Primary endpoints included ADAS-Cog v14 and ADCOMS. Secondary endpoints included RAVLT, CDR-SOB, MMSE, NPI and NTB.
All these endpoints are robust measures of cognition in AD trials and we believe the design of the trial was sound. Actinogen indicated it will conduct detailed analysis of the data, looking for potential trends. In addition, Actinogen is close to reporting data from its target occupancy and XanaHES higher dose (20mg and 30mg) safety studies. The company expects the insights from these and the XanADu studies will allow the design of a path forward for Xanamem. For example, this could be a longer treatment study with higher dosing. Initial XanaHES results are expected by end of June 2019, when there could be more clarity on the future of Xanamem. For the time being, our forecasts and valuation is under review.
Business description
Actinogen Medical is an ASX-listed Australian biotech developing its lead asset Xanamem, a specific 11β-HSD1 inhibitor designed to treat cognitive impairment that occurs in chronic neurodegenerative and metabolic diseases. The primary indication is cognitive impairment in mild AD patients and results from the ongoing Phase II XanADu trial are expected to be announced Q219.