Attacking Hep C via ITHACA and SPARTA
Achillion (NASDAQ:ACHN) continues to progress its Hep C portfolio though clinical trials with highly encouraging data from its Phase II proxy study with ACH-3102 paving the way for the initiation of more key combination Hep C trials this year. We maintain that Achillion is an attractive candidate for collaboration with a company interested in exploiting the considerable HCV market, but which lacks the in-house components necessary for a competitive HCV oral drug.
Triple combi could be epitome of Hep C treatments
ACH-3102 and Gilead (NASDAQ:GILD) sofosbuvir showed an SVR12 (sustained viral response in 12 weeks) after six weeks of therapy, beating the next best and currently marketed Hep C combination treatment with SVR12 after 8-12 weeks of treatment. The data enable the advancement of Achillion’s proprietary drugs into trials supporting the launch of a potent triple cocktail (ACH-3102+ACH-3422+sovaprevir) aiming to reach an SVR12 of less than six weeks. We currently forecast peak sales of $3.4bn for Achillion in Hep C following a triple combination launch in 2018.
Hepatitis C market on watch
Recently announced heavy discounting of Hep C drugs, as well as high cure rates, have called into question long-term market potential in Hep C. However, we expect the sheer size of the market – sales of Hep C treatments tipped $14bn in 2014 and are widely expected to surpass $20bn in the short term – low rates of diagnosis and heavy patient back logs to shore up demand well into the next decade.
Complementary Factor D enters Phase I this year
All eyes are on Hep C, although we note Achillion’s promising novel Factor D programme with a lead oral compound set to enter the clinic this year. Research thus far has focused on paroxysmal nocturnal hemoglobinuria (PNH), for which the only approved treatment is Alexion’s Soliris (2014 sales $2.2bn), which has significant limitations including intravenous administration and a black box warning.
Valuation: $1.85bn
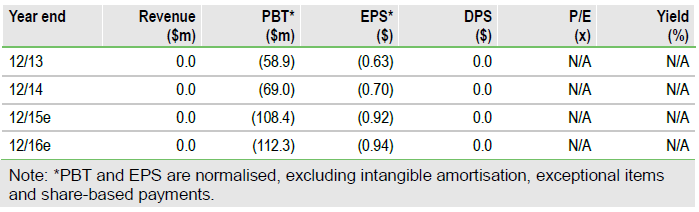
Our fair value for Achillion of $1.85bn, adjusted from $1.86bn ($15.8/share from $18.5/share), is derived from an NPV analysis of the company’s potential triple combination treatment in HCV, forecasting a 2018 launch. The Phase I start of a Factor D compound, targeted for later this year, would trigger inclusion of the programme in our forecasts, adding an estimated risk-adjusted $125m to our valuation.
To Read the Entire Report Please Click on the pdf File Below