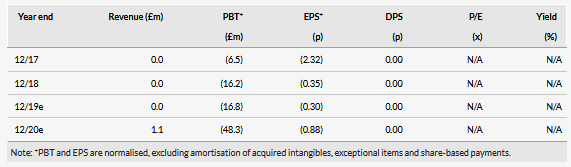
Acacia (NASDAQ:ACTG) has announced that the FDA has accepted the resubmitted NDA for key asset BARHEMSYS for post-operative nausea and vomiting (PONV). As expected, the FDA has categorised it as a Class 2 resubmission and set a PDUFA date of 26 February 2020. Prompt launch execution will be critical once approved, and Acacia’s US sales team is positioning itself for launch in H120. Managing cash burn during the intervening period is essential as we forecast that Acacia will need to raise c £40m in H120 (following potential approval) to fund operations, with additional future funding dependent on sales execution. We retain our previous forecasts (GBP denominated) and valuation of €631m.
Acacia’s lead asset, BARHEMSYS (repurposed amisulpride), is being developed for the management of PONV. Following two complete response letters (CRLs) from the US FDA during the past 12 months, deficiencies with Acacia’s previous chosen contract manufacturing organisation (CMO) had been highlighted. Acacia has nominated an alternative CMO which has a track record in both manufacturing amisulpride and undergoing regular, successful FDA inspections (c 60% of its current production is already US bound). With Acacia’s commercial focus concentrated on the US, it has changed its presentation currency from GBP to US$. We will reflect this in our financial model in due course, but retain our previous forecasts (GBP denominated) and valuation of €631m in the interim.
Business description
Acacia Pharma is a hospital pharmaceutical company focused on the development and commercialisation of new nausea and vomiting treatments for surgical and cancer patients. Its main product, BARHEMSYS, is for the treatment of PONV and could be approved by the FDA in 2020.