Synta looks set to start the Phase III stage of the GALAXY study of ganetespib in second-line non-small cell lung cancer (NSCLC) with broad entry criteria, after interim data suggested more consistent activity than was previously expected across key sub-groups. Synta has confirmed that the Phase III stage will enrol around 500 patients. A decision to enrol all comers with adenocarcinoma, if confirmed, is a potentially significant value-generating event for Synta, as our valuation currently assumes it will target a substantial minority of patients (ie 25-40%).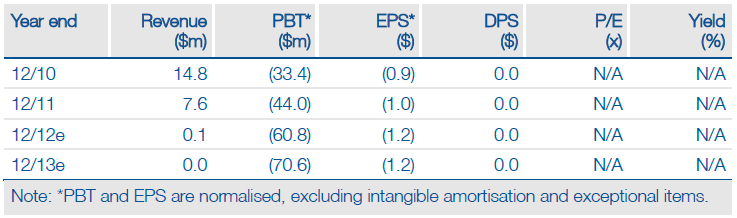
KRAS/LDH Data Leads To Broader Use
Interim data from the first stage of the Phase IIb/III study presented at ESMO suggest ganetespib has more consistent activity across the KRAS mutant/wild type and elevated/normal LDH subgroups than was indicated by prior data. Synta’s strategy may thus shift away from one that targets the poor prognosis but higher-responder subgroups to one addressing the broader adenocarcinoma population.
Clear OS Separation After 100 Days
The data show a strong evidence of an overall survival benefit, although this is evident only after around 100 days. This supports a new hypothesis that suggests if ganetespib can be given for a sufficient period, it may favourably change the biology of the tumour. This suggests that one possible strategy may be to refine the Phase III stage of GALAXY to target better prognosis patients -- those likely to be treated for 100 days or more -- possibly by excluding ones with metastases on entry or where diagnosis was made within six months.
CHIARA Study In ALK+ NSCLC
The separate CHIARA Phase II study of ganetespib as monotherapy in ALK-positive patients gives Synta two potential registration strategies directed at NSCLC. The Phase III start for ganetespib will confirm ganetespib’s position as the most advanced Hsp90 inhibitor in the field.
Valuation: $702m Or $11.40/share
For the time being we maintain our valuation of the R&D pipeline, excluding cash, at $702m, equivalent to $11.40 per share (basic), although this still assumes a 50% risk adjustment for all indications. The Phase III stage initiation and a strategy of addressing the broader population would add a significant contribution to the value.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Synta Pharmaceuticals GALAXY Interim Results
Published 10/11/2012, 11:35 AM
Updated 07/09/2023, 06:31 AM
Synta Pharmaceuticals GALAXY Interim Results
Ganetespib Plan Takes Shape
Latest comments
Loading next article…
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.