Synta has disclosed the much-anticipated data that supported a decision to go ahead with the Phase III stage of its GALAXY study of ganetespib in second-line, non-small cell lung cancer (NSCLC). The data show encouraging signs of efficacy, including a 2.5 to 3-fold improvement in PFS in two subgroups of adenocarcinoma, namely those with mutant KRAS and with elevated LDH. Improvements were seen in PFS and OS (with separation of the Kaplan-Meier curves) in the all adenocarcinoma group, but not in non-adeno. However, the decision not to disclose the statistical data on these results, seemingly for good reasons, panicked the market.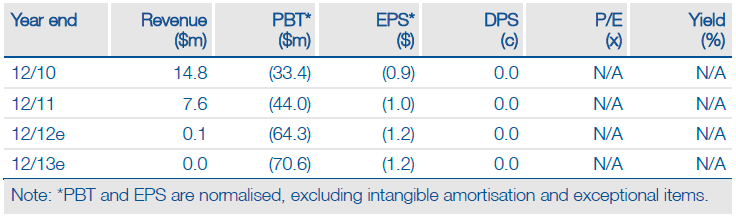
2.5x increase In PFS In KRAS And 3x In High LDH
The data show a solid increase -- from 1.4-1.6 to 4.2 months -- in progression free survival (PFS) in two key subgroups of adenocarcinoma: elevated LDH and mutant KRAS, both of which correlate with poor response. No effect, however, was seen in the non-adenocarcinoma group. The study now recruits adenocarcinoma only and will render more mature data in Q3. Synta intends to present the interim data at a medical conference later in the year, probably at ESMO (28 September-2 October).
But The Market Takes The (Lack Of) p Badly
Synta’s decision not to disclose statistics (p values or hazard ratios) for the subgroups (it cited a number of reasons including the immaturity of the data and the small size of the subgroups) spooked the market, triggering a surprise 33% sell-off in the stock. The share price had, however, risen strongly in anticipation of the data and has since started to recover.
CHIARA Study Aiming For Clear Signal In ALK+
The separate CHIARA Phase II study of ganetespib, as monotherapy, in ALK-positive NSCLC, gives Synta two registration directed strategies in NSCLC, confirming ganetespib’s position as the most advanced Hsp90 inhibitor.
Valuation: $702m Or $12.20/share
We maintain our valuation of the R&D pipeline of $702m, equivalent to $12.20 per share (basic), without adjusting for cash. By comparison, Synta’s EV is currently $268m, highlighting an attractive investment case. Furthermore, we continue to assume a 50% risk adjustment for all indications (lower than is typical for a Phase III asset), pending definitive data from the Phase IIb stage.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Synta Pharma: GALAXY Interim Results
Published 07/03/2012, 11:15 AM
Updated 07/09/2023, 06:31 AM
Synta Pharma: GALAXY Interim Results
Early, But Positive (As Expected)
Latest comments
Loading next article…
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.