ImmunoGen, Inc. (NASDAQ:IMGN) announced that the FDA has advised to conduct a new single-arm pivotal study, which can support accelerated approval for its lead candidate, mirvetuximab soravtansine, in ovarian cancer. We note that the FDA had recommended a new monotherapy study on the candidate in May, following the failure of phase III study – FORWARD 1.
Based on the FDA’s guidance, the company is planning to initiate a pivotal study, SORAYA, in patients with platinum-resistant ovarian cancer whose tumors express high or medium levels of FR alpha and who have received prior treatment with Roche’s (OTC:RHHBY) Avastin (bevacizumab). The company is expected to start enrolling patients in the SORAYA study by the next quarter, with top-line data expected to be available by mid-2021. The company is anticipating to file a regulatory application in the second half of 2021, seeking accelerated approval for mirvetuximab soravtansine, if the study is completed successfully.
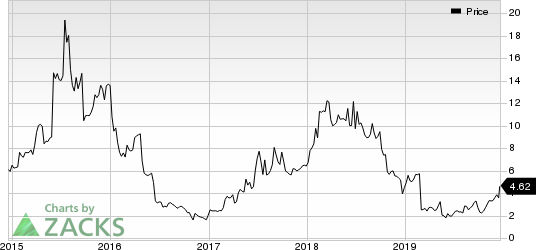
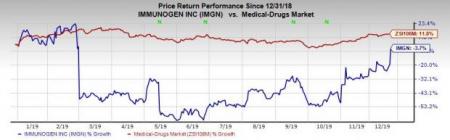
Shares of ImmunoGen gained 18.8% on Dec 17 following the announcement. Investors cheered the news as a single-arm, pivotal study is likely to reduce the time to get an approval for mirvetuximab soravtansine. However, the company’s shares have decreased 3.7% so far this year against the industry’s increase of 11.8%.
Please note that the company announced in March that its FORWARD 1 study failed to meet the primary endpoint of progression-free survival (“PFS”). The FORWARD 1 study evaluated the efficacy of mirvetuximab soravtansine as a single-agent therapy compared to chemotherapy for the treatment of patients with platinum-resistant ovarian cancer whose tumors express high or medium levels of FR alpha and who have received up to three prior treatment regimens. However, the overall response rate (“ORR”) for the candidate was higher tin the study compared to patients who were treated with chemotherapy.
The SORAYA study will evaluate the candidate in patients with platinum-resistant ovarian cancer whose tumors express high levels of FRα and have received up to three prior treatments, including at least one with Avastin. However, the primary endpoint of the study will be ORR instead of PFS as in FORWARD 1 study.
Meanwhile, the company stated on its third-quarter earnings call that it has met with the FDA to review the design of a phase III study – MIRASOL – which will compare mirvetuximab soravtansine head-to-head with single agent chemotherapy in platinum-resistant ovarian cancer patients with high folate receptor alpha expression. The company expects to initiate enrollment in the study by the year end. It is planning to use this study as a confirmatory study, which will support continued approval for mirvetuximab soravtansine, if it gets accelerated approval based on SORAYA study data. Top-line data from MIRASOL study is anticipated in the first half of 2022.
A phase Ib/II FORWARD II study is evaluating mirvetuximab soravtansine in combination with Avastin or Merck’s (NYSE:MRK) Keytruda for treating cancerous tumors especially with medium or high FRα expression levels.
Apart from mirvetuximab soravtansine, the company is also developing another pipeline candidate, IMGN632, in several early- to mid-stage studies as monotherapy or in combination with Celgene’s Vidaza or AbbVie (NYSE:ABBV) /Roche’s Venclexta for treating acute myeloid leukemia and other oncology indications.
Zacks Rank
ImmunoGen currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
7 Best Stocks for the Next 30 Days
Just released: Experts distill 7 elite stocks from the current list of 220 Zacks Rank #1 Strong Buys. They deem these tickers “Most Likely for Early Price Pops.”
Since 1988, the full list has beaten the market more than 2X over with an average gain of +24.6% per year. So be sure to give these hand-picked 7 your immediate attention.
See 7 handpicked stocks now >>
AbbVie Inc. (ABBV): Free Stock Analysis Report
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
Merck & Co., Inc. (MRK): Free Stock Analysis Report
ImmunoGen, Inc. (IMGN): Free Stock Analysis Report
Original post
Zacks Investment Research