Aimmune Therapeutics, Inc. (NASDAQ:AIMT) announced that the FDA has approved its oral immunotherapy Palforzia [Peanut (Arachis hypogaea) Allergen Powder-dnfp] for the treatment of patients with peanut allergy. Following this nod, Palforzia becomes the first approved therapy for addressing the given patient population.
Palforzia is designed to reduce the incidence and severity of allergic reactions including anaphylaxis, which may occur due to accidental exposure to peanut. The drug is to be administered in conjunction with a peanut-avoidant diet and only in patients with a confirmed diagnosis of peanut allergy.
Per the company, initial dose escalation may be administered to patients aged from four to 17 years and up-dosing and maintenance may be continued in patients aged four years and above. The medicine is available only through a Risk Evaluation and Mitigation Strategy (REMS), which requires both the prescribing physician and the respective patient to be enrolled in the REMS before starting treatment.
However, the prescribing information for Palforzia comes with a boxed warning.
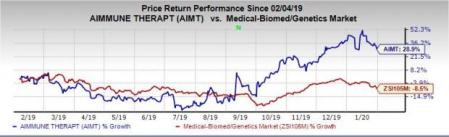
Shares of Aimmune were up 22% in pre-market trading on Monday. In fact, the stock has rallied 28.9% in the past year against the industry’s decline of 8.5%.
Notably, the biologics license application (BLA) for Palforzia was based on the safety and efficacy data from seven clinical studies including the pivotal phase III PALISADE and RAMSES studies.
In September 2019, the Allergenic Products Advisory Committee (APAC) convened by the FDA voted in favor of Palforzia for use in children and teens with peanut allergy. The APAC voted in favor of the drug's effectiveness in the ratio of 7 to 2. The committee also voted in the ratio of 8 to 1 backing the safety data in conjunction with additional safeguards, which are enough to support the use of Palforzia.
The marketing authorization application (MAA) for Palforzia is currently under review in Europe to treat peanut allergy in children and adolescents aged from four to 17 years. A decision from the European Medicines Agency (EMA) is expected in the second half of 2020.
Peanut allergy is one of the most common food allergies, which affects more than 1.6 million children and teens in the United States alone. Hence, this approval should boost growth prospects of this clinical-stage biopharmaceutical company.
Zacks Rank & Stocks to Consider
Aimmune currently carries a Zacks Rank #4 (Sell).
Better-ranked stocks in the biotech sector include Xenon Pharmaceuticals Inc. (NASDAQ:XENE) , Denali Therapeutics Inc. (NASDAQ:DNLI) and Kala Pharmaceuticals, Inc. (NASDAQ:KALA) , all sporting a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Xenon Pharmaceuticals’ loss per share estimates have narrowed 12% for 2020 over the past 60 days. The stock has soared 70.2% in the past year.
Denali’s loss per share estimates have narrowed 1.25% for 2020 over the past 60 days. The stock has jumped 20.9% in the past year.
Kala Pharmaceuticals’ loss per share estimates have narrowed 0.9% for 2020 over the past 60 days.
Free: Zacks’ Single Best Stock Set to Double
Today you are invited to download our latest Special Report that reveals 5 stocks with the most potential to gain +100% or more in 2020. From those 5, Zacks Director of Research, Sheraz Mian hand-picks one to have the most explosive upside of all.
This pioneering tech ticker had soared to all-time highs and then subsided to a price that is irresistible. Now a pending acquisition could super-charge the company’s drive past competitors in the development of true Artificial Intelligence. The earlier you get in to this stock, the greater your potential gain.
Aimmune Therapeutics, Inc. (AIMT): Free Stock Analysis Report
Xenon Pharmaceuticals Inc. (XENE): Free Stock Analysis Report
Denali Therapeutics Inc. (DNLI): Free Stock Analysis Report
Kala Pharmaceuticals, Inc. (KALA): Free Stock Analysis Report
Original post
Zacks Investment Research
