On Apr 4, we issued an updated research report on Ironwood Pharmaceuticals, Inc. (NASDAQ:IRWD) . This biopharmaceutical company, based in Cambridge, MA, is focused on the development and commercialization of treatments primarily addressing gastrointestinal (“GI”) diseases. The company’s sole marketed drug, Linzess, is indicated for the treatment of irritable bowel syndrome with constipation (IBS-C) and chronic idiopathic constipation (“CIC”).
We remind investors that Ironwood co-develops and co-commercializes Linzess and equally shares Linzess U.S. collaboration profits/losses as well as development costs with Allergan (NYSE:AGN) .
In April 2018, the company has separated its Soluble Guanylate Cyclase (sGC) business into a new entity named Cyclerion Therapeutics.
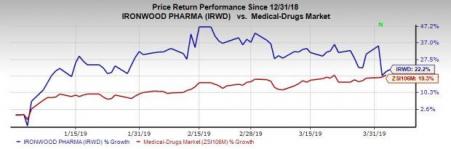
Shares of the company have rallied 22.2% so far this year compared with the industry’s rise of 19.3%.
Linzess Remains Impressive
Sales of Linzess were encouraging in 2018 as well. Total revenues for 2018 increased 16.2% year over year to $346.6 million primarily on the back of strong sales of Linzess. The drug’s sales rose more than 9% in 2018. Prescriptions filled in 2018 were also up almost 7% year over year.
In January 2019, Linzess received approval in China for IBS-C. The company along with its partner, AstraZeneca (NYSE:AZN) , will launch the drug in China in the second half of 2019. This is likely to boost Ironwood’s top line.
The company also earns by selling linaclotide (Linzess) active pharmaceutical ingredient (API) to Japan-based Astellas Pharma. Ironwood Pharma generated almost $70.4 million from this transaction in 2018 compared with $29.7 million in the year-ago period. Astellas Pharma started commercializing Linzess in Japan for IBS-C in early 2017.
Pipeline Progress
Ironwood and Allergan are conducting a phase III study to evaluate Linzess in additional abdominal symptoms including bloating and discomfort in adult patients with IBS-C. A label expansion in pediatric patients is also being planned.
A phase IIb study is evaluating MD-7246, a delayed-release formulation of Linzess, as a treatment for all subtypes of IBS, including IBS-C, IBS with diarrhea and IBS-mixed. The company is also developing another GI candidate, IW-3718, in phase III studies for treating persistent refractory gastroesophageal reflux disease (“GERD”).
Cyclerion Spin-off
On Apr 1, Ironwood completed the previously announced separation of its sGC business and transferred the sGC pipeline for the treatment of serious and orphan diseases to newly-formed Cyclerion. This will help Ironwood to focus on its GI pipeline.
Ironwood started reducing its workforce following the separation, which is likely to boost margins going forward. The company also reduced employees in 2018 related to separation of business as well as termination of lesinurad license with AstraZeneca. Although the separation of business will increase selling, general and administrative expense, research and development expenses are likely to decrease due to transfer of sGC pipeline in 2019.
Our Take
The spin-off of sGC pipeline and focus on developing GI pipeline is a positive for Ironwood. It will help the company invest more resources for the development of its GI candidate. Label expansion of Linzess will further boost the prospects of the drug.
However, we note that Ironwood is primarily dependent on Linzess for revenues. Any setback related to manufacturing or commercialization of the drug will impact the company’s top line. The competition in target markets for Linzess is also increasing. Other available products include Takeda Pharma’s (NYSE:TAK) Amitiza, Takeda’s Motegrity and Synergy’s (now a part of Bausch Health Companies) Trulance, Linzess also faces competition from laxatives and over-the-counter fiber supplements. Pricing pressure also hurts revenues from Linzess. Moreover, Ironwood’s GI pipeline is years away from commercialization.
Zacks Rank
Ironwood currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Zacks' Top 10 Stocks for 2019
In addition to the stocks discussed above, would you like to know about our 10 finest buy-and-holds for the year?
Who wouldn't? Our annual Top 10s have beaten the market with amazing regularity. In 2018, while the market dropped -5.2%, the portfolio scored well into double-digits overall with individual stocks rising as high as +61.5%. And from 2012-2017, while the market boomed +126.3, Zacks' Top 10s reached an even more sensational +181.9%.
Allergan plc (AGN): Free Stock Analysis Report
AstraZeneca PLC (AZN): Free Stock Analysis Report
Ironwood Pharmaceuticals, Inc. (IRWD): Free Stock Analysis Report
Takeda Pharmaceutical Co. (TAK): Free Stock Analysis Report
Original post
Zacks Investment Research