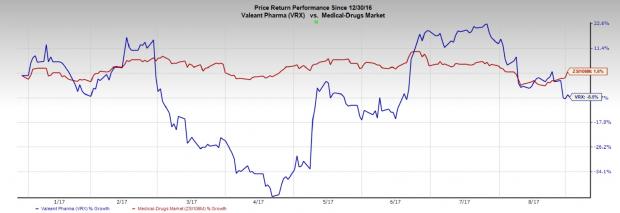
Shares of Valeant Pharmaceuticals International, Inc. (NYSE:VRX) have declined 7.6% so far in 2017 against 1.6% gain of the industry. Shares fell further 6.9% on Aug 29.
Once an acquisition giant, Valeant has been caught up in various controversies since October 2015 due to price hike of specialty drugs, erroneous financial reporting and termination of contracts with Philidor Rx Services.
Following a substantial decline in Valeant’s share price in the first quarter of 2016, Bill Ackman and Steve Fraidin joined the board in March 2016 as part of Pershing Square’s effort to stabilize the company. Thereafter, Joseph C. Papa took charge as the new CEO. The new management took various efforts to turnaround the company.
Even though it is still early to comment on the rebuilding process but the company’s efforts to sell non-core assets and lower debt is commendable. Valeant sold its equity interests in Dendreon Pharmaceuticals, Inc. to China-based Sanpower Group Co. Ltd. Valeant has also decided to sell Obagi. The company reduced debt by $4.8 billion and expects to meet its target of $5 billion debt reduction ahead of schedule. The divestiture of non-core assets should help the company streamline its product portfolio and focus better on core areas of dermatology and lower debt.
However, weakness in the dermatology segment persists and is expected to impact the top line.
Valeant recently announced that the FDA confirmed to issue a Voluntary Action Indicated (VAI) inspection classification for its Bausch + Lomb manufacturing facility in Tampa, FL as part of a forthcoming Establishment Inspection Record for the facility. With this confirmation, manufacturing uncertainties related to the current and upcoming regulatory submissions will be eliminated for products manufactured at the facility.
In August 2017, the company received a Complete Response Letter (CRL) from the FDA regarding the New Drug Application (NDA) for latanoprostene bunod ophthalmic solution, 0.024%, an investigative intraocular pressure lowering single-agent eye drop for patients with open angle glaucoma or ocular hypertension. The CRL from the FDA refers to a Current Good Manufacturing Practice (CGMP) inspection at Bausch + Lomb's manufacturing facility in Tampa, FL.
On a positive note, the company received FDA filing acceptance for the NDA for Plenvu (NER1006), a novel, low volume polyethylene glycol-based bowel preparation for colonoscopies. Earlier, the FDA approved its new psoriasis treatment, Siliq. The company had an agreement with AstraZeneca (NYSE:AZN) for Siliq but the agreement has been amended.
Although the approval of new drugs brings some hope. We believe it is still a long road ahead for Valeant before the investor faith can be restored. Earlier in the year, Bill Ackman, chairman of Perishing Square (NYSE:SQ) Holdings, Ltd stated that investing in Valeant was a big mistake on his part.
Zacks Rank & Key Picks
Valeant currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the health care sector are Aduro Biotech, Inc. (NASDAQ:ADRO) and Gilead Sciences, Inc. (NASDAQ:GILD) . Both stocks carry a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Aduro Biotech’s loss per share estimates narrowed from $1.46 to $1.36 for 2017 over the last 30 days. The company delivered positive earnings surprises in two of the four trailing quarters with an average beat of 2.53%.
Gilead’s earnings per share estimates increased from $7.92 to $8.53 for 2017, over the last 30 days following strong results in the second quarter. The company delivered positive earnings surprises in three of the trailing four quarters, with an average beat of 8.18%.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple (NASDAQ:AAPL) sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 27 billion devices in just 3 years, creating a $1.7 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 6 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2020. Click here for the 6 trades >>
Astrazeneca PLC (LON:AZN): Free Stock Analysis Report
Gilead Sciences, Inc. (GILD): Free Stock Analysis Report
Aduro Biotech, Inc. (ADRO): Free Stock Analysis Report
Valeant Pharmaceuticals International, Inc. (VRX): Free Stock Analysis Report
Original post
Zacks Investment Research
