FDA approval for Amitiza (SCMP) to treat opioid-induced constipation (OIC) in adults with chronic, non-cancer pain has sparked a 40% share price rally. OIC affects ~2.5m patients in the US, and with Amitiza the only available therapy and safety concerns over potential competitors, OIC is a significant new market opportunity. We model $215m peak US sales in OIC and have increased our total US peak sales estimate to $665m (vs $590m), on the back of strong Q412 sales in other constipation disorders. We therefore raise our overall valuation of Sucampo to $420m, or $10.00 per share (vs $335m, $8/share). Sucampo is now trading close to its fair value, although upside exists in Amitiza’s commercial potential in Europe.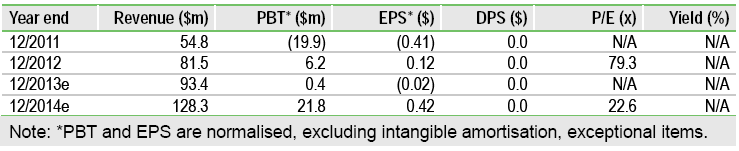
Energising opportunity
Expansion of Amitiza’s label to OIC provides US partner Takeda with a great opportunity to energise its promotion of the drug, which had been criticised by Sucampo and was the subject of legal disputes between the companies. With the FDA’s safety concerns over mu opioid antagonists potentially stalling a number of competitors (Relistor, naloxegol, bevenopran and TD-1211), Amitiza may have a free run at OIC, an easier market to target (than chronic/irritable bowel-related constipation). OIC patients are actively managed by physicians more aware of treatment options for constipation and more likely to prescribe a drug like Amitiza (vs OTC laxatives), especially if it is approved for multiple constipation types. FDA approval for OIC triggers a $10m milestone payment from Takeda.
Amitiza absorbing Linzess challenge
Our analysis of prescription trend data suggests that, so far, Amitiza has largely been unaffected by the US launch of Linzess (Ironwood/Forest) in December 2012, Amitiza’s first direct competitive threat since Zelnorm was withdrawn in 2007. Indeed, with Ironwood reporting that 60% of Linzess prescriptions are for new patients switching from OTC laxatives, the entrance of Linzess would appear to be expanding the market for the use of Rx drugs in constipation (currently <10%).
Valuation: OIC and Amitiza Rx boost to $10.00/share
Removing Amitiza’s risk-adjustment for OIC, increasing peak US sales, applying an estimated tiered-royalty rate on increased sales, and a better-than-expected margin on Amitiza transfer revenues to AbbVie for sale in Japan, boosts our overall valuation of Sucampo to $420m ($335m), or $10.00 ($8.00) per share. Sucampo’s >40% share price gain to $9.51 on the back of Amitiza’s OIC approval brings the stock close to our fair valuation. However, we acknowledge upside potential as our valuation does not currently include Amitiza’s commercial opportunity in Europe (peak sales $100-150m), and further line extensions for Amitiza and Rescula.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Sucampo Pharmaceuticals: Energising Opportunity
Published 05/01/2013, 08:32 AM
Updated 07/09/2023, 06:31 AM
Sucampo Pharmaceuticals: Energising Opportunity
A run on Amitiza
Latest comments
Loading next article…
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.