The corporate split is progressing well. The Polish Financial Supervisory Authority has approved the Selvita (WA:SLVP) CRO Prospectus as part of listing on the Warsaw Stock Exchange and the shareholders' meeting approved the split. The current entity will continue as Ryvu Therapeutics, a newly introduced brand, and will fully focus on innovative drug discovery and development. The process should complete in October 2019. We believe both businesses have sufficient momentum to continue as standalone companies. Our updated valuation stands at PLN1.46bn or PLN91.5/sh.
Sound rationale...
We believe a split into two companies – services (named Selvita) and oncology R&D (named Ryvu Therapeutics) – will be beneficial as it will allow investors to adjust their exposure to specific risk-return investments. As a standalone entity, the fast-growing and profitable Services segment will be able to diversify its capital structure, lower overall costs of capital and complement organic growth with acquisitions. The innovative R&D company will become a classic drug developer, and therefore will have the benefits of a biotech business model: flexible capital allocation to R&D and direct access to capital markets.
…for a well-timed corporate split
Selvita’s most clinically advanced asset SEL24 is out-licensed to Menarini Group (known as MEN1703). Therefore, the new oncology company will focus on the clinical development of the second most-advanced asset SEL120, a first-in-class, selective CDK8 inhibitor, and on advancing the broad discovery and preclinical pipeline. Menarini is progressing with the Phase I/II trial with SEL24, while Selvita’s Phase Ib study with SEL120 has just been initiated (first patient dosed on 6 September). Therefore, data readouts (key catalysts) from both trials should be released over 2020, making the corporate split well timed (Q419).
Maturing R&D pipeline will underpin Selvita Oncology
Other earlier-stage projects include a best-in-class dual A2A/B receptor antagonist, small molecule STING agonist for systemic administration, an HPK1 inhibitor and first-in-class SMARCA2 inhibitor for treating SMARCA4 mutated cancers. Selvita Oncology (Ryvu Therapeutics) also has numerous undisclosed projects at earlier stages and we expect these to ‘feed’ the preclinical and clinical pipeline.
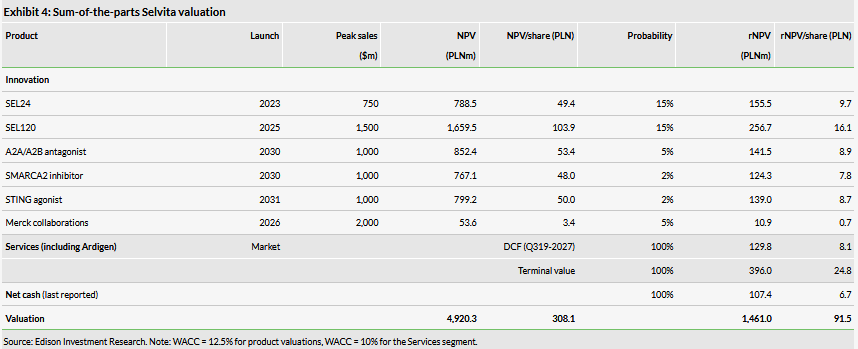
Valuation: PLN1.46bn or PLN91.5/share
Our valuation of Selvita is higher at PLN1.46bn or PLN91.5/sh, vs PLN1.34bn or PLN83.9/sh previously, which is mainly due to increased probabilities of success for SEL120 and A2A/A2B antagonist projects. The CRO business corresponds to 36% (DCF) of the total valuation, R&D segment 57% (rNPV) and net cash 7%.
Business description
Selvita is an R&D and drug discovery services company. It operates three business segments: Innovations Platform (internal R&D pipeline), Research Services (medicinal chemistry/biology, biochemistry) and Ardigen (a spin-out bioinformatics company, 52% owned). The lead asset is wholly owned SEL120, which is being studied in a Phase Ib clinical trial. Selvita is also focusing on its other projects in a broad pipeline dedicated to oncology.
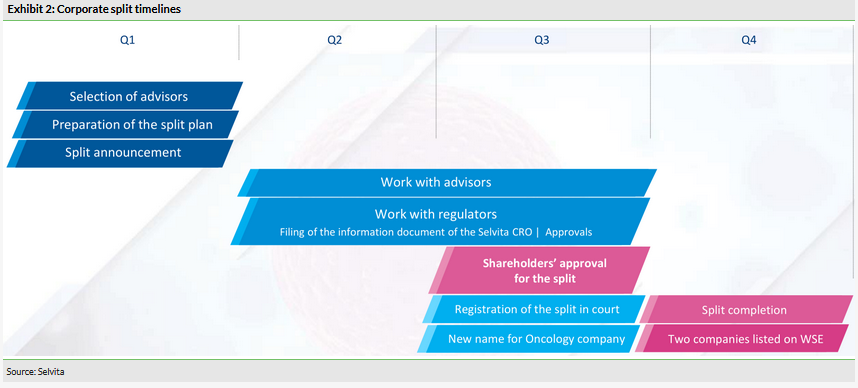
Corporate split update
Selvita is in the regulatory process to split into two listed companies. The current entity will continue as Ryvu Therapeutics, a newly introduced brand, and will fully focus on innovative drug discovery and development. Selvita’s CRO business will be spun out and will keep the Selvita brand. The corporate split is expected to complete in October 2019 and is intended to accelerate the development of both businesses. The innovative oncology company will control the current R&D pipeline of small molecules, as well as shares in NodThera. As a standalone business, the services company will seek to maximise its sales (2014–18 CAGR of 30%) and profitability via organic growth and the acquisition of complementary drug discovery service providers. As a profitable company, it will also have easier access to external financing. To recap, the details of the split include:
Selvita Oncology will remain as the listed entity and will be renamed Ryvu Therapeutics, while Selvita Contract Research Organization will be spun out and will keep the Selvita brand.
The current shareholding structure of Selvita should be maintained by both new companies. For each existing share in Selvita, the shareholders will own one share in the oncology R&D company and one share in the services company. Both will be listed on the Warsaw Stock Exchange.
Around 180 employees will be part of the innovative oncology company and the remaining 420 (including Ardigen) will join the services company.
Current Selvita CEO Pawel Przewiezlikowski will be CEO of the oncology company and COO Boguslaw Sieczkowski will become CEO of the contract research company (both are co-founders of Selvita).

All Ardigen (Bioinformatics spinoff) shares owned by Selvita will be allocated to the services company and all NodThera shares owned by Selvita will be allocated to Ryvu Therapeutics.
Oncology R&D update
We provided a detailed overview of Selvita’s R&D pipeline in our last outlook report published in January 2019, including the two most advanced assets in clinical development: SEL24, a dual PIM/FLT3 inhibitor, and wholly owned SEL120, a selective CDK8 inhibitor. Selvita has recently revised how the assets are arranged into platforms. Only discovery and preclinical projects are now assigned to one of the two Immunooncology & immunometabolism or Synthetic lethality platforms. The clinical stage assets (SEL24 and SEL120) and collaborations with Merck KGaA are run as separate programmes.
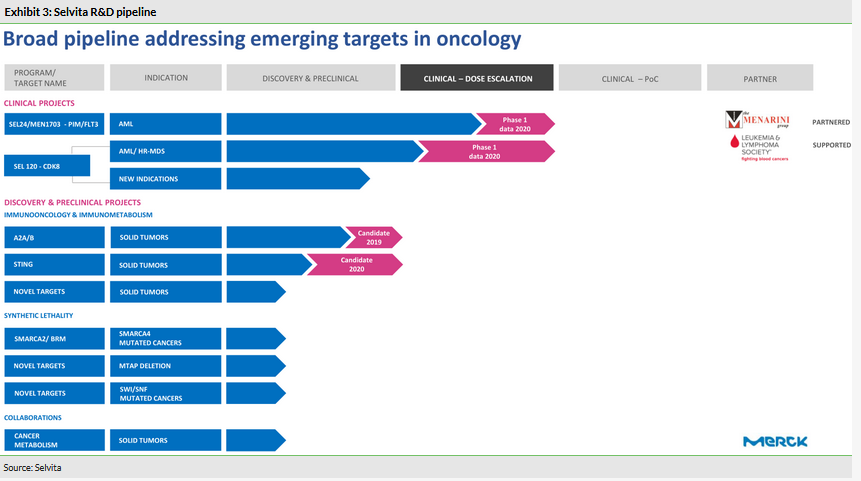
SEL24/MEN1703
The Phase I/II trial (n=86) with SEL24/MEN1703, a dual inhibitor of PIM and FLT3 kinases, in acute myeloid leukaemia (AML) patients is progressing according to plan. The expected advantage of SEL24/MEN1703 versus other FLT3 inhibitors, such as quizartinib (Daiichi Sankyo) or gilteritinib (Astellas), is that it could potentially be used in patients regardless of FLT3 mutational status (for a detailed discussion, see our last outlook report). The latest update on the programme came from partner Menarini, which now solely runs the study in the US. This included two recent poster presentations at two high-profile clinical conferences organised by the ASCO and the EHA in June 2019. As the Phase I/II study is still enrolling patients, no data have been released so far and the conference presentations focused on the design of the study, the rationale and the enrolment progress. Menarini indicated the trial will be expanded to involve approximately 40 centres in the US and Europe (until now only five centres were active, located exclusively in the US).
SEL120
Following the out-licensing of SEL24, SEL120, a selective CDK8 inhibitor, is the most advanced asset being developed by Selvita. The FDA approved an investigational new drug (IND) application in March 2019 in patients with AML or high-risk myelodysplastic syndrome. The first-in-human Phase Ib study with SEL120 is ongoing, with the first patient dosed in early September 2019 at an investigation site in the US. In total five sites are planned to be activated in the US by end of this year. Preliminary interim data will be shared by the end of 2020 and will include patients who complete the treatment before Q320. The initial focus on haematological malignancies is supported by the Leukemia and Lymphoma Society (LLS) and Selvita has received US$1.5m in research funding in total since the collaboration was established in August 2017. Selvita is also evaluating the potential of SEL120 in other haematological malignancies, including lymphomas, and in solid tumours. The company also plans preclinical combination studies of SEL120 with a number of other cancer treatment agents, such as targeted therapies, chemotherapy and checkpoint inhibitors (CPIs).
Discovery and preclinical projects
Immunooncology & immunometabolism platform
The most advanced disclosed asset within the Immunooncology & immunometabolism platform is a best-in-class dual A2A/A2B receptors antagonist. The targets belong to the so-called adenosine pathway and adenosine is one of the major microenvironmental agents that allows tumour to avoid recognition by the immune system. Selvita’s compound is highly active (picomolar activity range). In the in vivo studies presented at this year’s AACR conference in April, it demonstrated >90% tumour growth inhibition in combination with a checkpoint inhibitor (anti-CTLA4) in a CT26 syngeneic mouse model. In another melanoma mouse model (B16-F10), where the animals are completely resistant to checkpoint inhibitor (anti-PD-1) treatment, the addition of Selvita’s A2A/A2B antagonist also demonstrated significant tumour growth inhibition. These findings support the strong rationale for the synergistic potential of combinations with checkpoint inhibitors. Selvita expects to select the drug candidate and start preclinical studies in Q419.
The second most advanced project is focused on discovering small molecule, direct STING agonists. STING receptor is a known mediator of the immune system, which when activated induces the expression of type I interferon and other T-cell recruitment factors. This results in the activation of dendritic cells, which act as antigen-presenting cells. The ultimate outcome is the specific immune response with ‘trained’ CD8+ T-cells attacking the cancer. The clinical opportunity for STING agonists is also combinations with CPIs, where the STING agonist can act as a primer increasing the effectiveness of CPIs or helping to overcome the resistance. Selvita aims to select a clinical drug candidate by the end of 2020.
Synthetic lethality platform
The disclosed targets within the Synthetic lethality platform include BRM/SMARCA2. In malignant cells with the mutated SMARCA4 gene, non-mutated SMARCA2 becomes essential. Therefore the inhibition of SMARCA2 causes cell death if there is an oncogenic mutation in the SMARCA4 gene. This concept of a ‘biological genetic flaw’ complemented by an intervention with a drug, which results in cell death, is known as synthetic lethality. This approach is very specific and potentially offers a good safety profile. Selvita is developing a first-in-class, selective SMARCA2 small molecule inhibitor and the available key data were reviewed in our last outlook report. When it comes to market opportunity, the non-small cell lung cancer (NSCLC) patient population could be the first clearly defined target as SMARCA4 is mutated in around 6–8% of cases. Targets of the other projects in synthetic lethality platform remain undisclosed.
Other projects
Other earlier-stage projects include an SHMT2 inhibitor (cancer metabolism one-carbon pathway), an HPK1 inhibitor (TCR/TLR pathway) and projects in synthetic lethality. Little information has been released from these projects for competitive reasons. We expect these early-stage projects will ‘feed’ the preclinical and clinical pipeline following the corporate split and as the lead discovery and clinical projects progress.
Financials
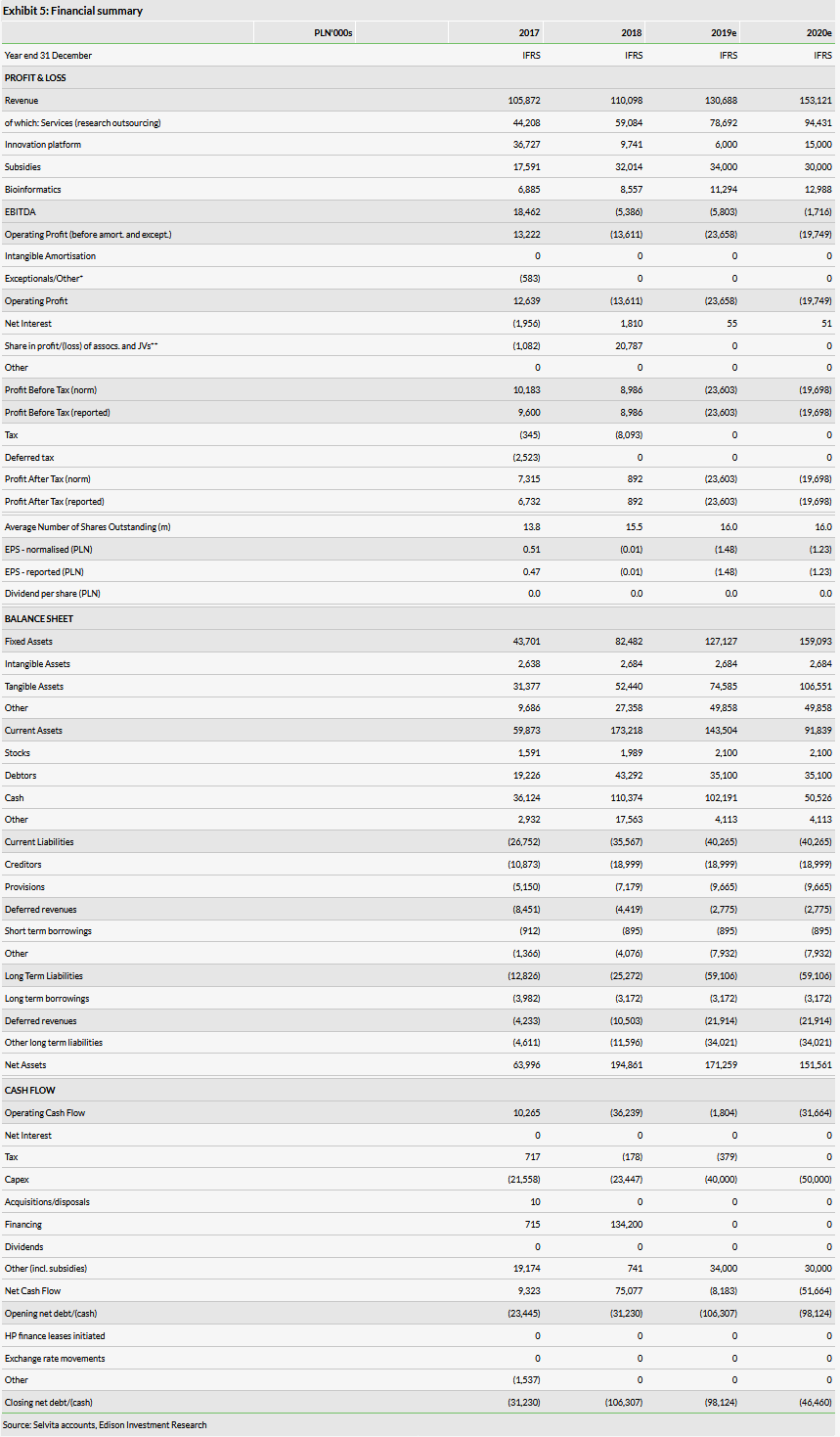
Selvita reported total H119 revenues of PLN62.7m (up 24% y-o-y), of which commercial revenues were PLN43.5m (up 17% y-o-y) and subsidies were PLN18.6m (up 44% y-o-y). The substantial increase in the latter is a result of the initiation of new innovative R&D projects. The updated order backlog was PLN116.3m in September 2019 (up 19% year-on-year).
The operating loss was PLN16.4m in H119 vs PLN2.1m in H118, reflecting more intensive R&D (Selvita’s R&D business segment). Selvita’s CRO business segment maintained a similar operating margin of 12% (14% in H118) mainly due to preparations for the corporate split and an increase in depreciation and amortisation following capital investments made in this segment in 2018. Selvita booked PLN8.9m in depreciation in H119 compared to PLN3.6m in H118. The increase was also a result of IFRS 16 changes to lease accounting. Depreciation was the only estimate we have adjusted in our model (Exhibit 5). Selvita reported cash and cash equivalents of PLN111.3m at end-H119 and PLN3.8m in debt.
Valuation
Our pre-split valuation of Selvita is somewhat higher at PLN1.46bn or PLN91.5/share, vs PLN1.34bn or PLN83.9/share previously. The CRO business now corresponds to 36% (DCF) of the total valuation, oncology drug development 57% (rNPV) and net cash 7%. The valuation upgrade was mainly a result of the revision of our rNPVs associated with the R&D assets:
For SEL120, which is now a clinical-stage asset, we have increased the probability of success to 15% from 10% following the initiation of the Phase Ib study.
For the A2A/A2B antagonist project, we have increased the probability of success to 5% from 2%. This project has made consistent progress and is now close to the IND-enabling studies.
For SEL24, we keep the probability of success at 15%, which is typical for Phase I assets. However, we find the update from partner Menarini, which will expand the number of centres enrolling patients from five to 40, particularly encouraging. This will substantially increase the rate of spending for the trial, so shows commitment from Menarini.
Overall, we maintain our valuation approach and assumptions discussed in detail in our last outlook report. We use risk-adjusted NPV models with a discount rate of 12.5% for Selvita’s R&D projects at various stages. Separately, we use DCF-based calculations with a discount rate of 10% to value the core drug discovery services business and research collaborations.
Newsflow over the next 12 months includes:
one new pre-clinical candidate selected from the internal discovery projects (Q419);
completion of the corporate split (early Q419);
SEL24 data from Phase I part (dose escalation) of the ongoing Phase I/II study (timing depends on Menarini); and
SEL120 interim data from the Phase Ib study (2020).