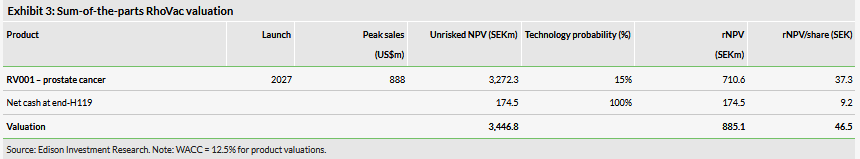
The past few months have been transformative for RhoVac ApS (TE:RHOVAC). The company completed a large rights issue, published final follow-up results from its Phase I/II trial with RV001 in prostate cancer and is about to enrol the first patient to a new controlled efficacy Phase IIb trial. On the corporate side of the business, a new CEO was appointed, bringing a wealth of business development expertise, a strategic fit given RhoVac’s priorities over the next two to three years. The recently received EU Horizon 2020 grant of €2.5m will not only provide a financial support from the EU Commission, but we also view it as a form of external validation. We have increased our valuation to SEK885.1m or SEK46.5/share (vs SEK37.2/share previously).
Final Phase I/II study follow-up results
On 4 July 2019, RhoVac released long-term follow-up results of the Phase I/II trial with RV001. In this study, safety and immunological response were assessed at three, six, nine and 12 months. Positive results after the three- and six-month follow-ups have been reported previously. The July announcement was the final 12-month update, which confirmed that treatment with RV001 was safe and elicited a longstanding immune response. RhoVac is now running a larger Phase IIb study to explore efficacy in the same patient group. The trial is expected to enrol the first patient imminently. Most of the study centres are in Europe, but RhoVac confirmed it will open several US centres. The final data are expected in H221.
New CEO appointed
RhoVac announced on 2 September 2019 it had appointed Anders Månsson as the new CEO. Former CEO, Anders Ljungqvist, will continue working as chief operating officer. Mr Månsson joined RhoVac in May 2019 as deputy CEO and chief business officer. We believe his expertise is a good fit strategically because RhoVac’s areas of focus over the next three years will be executing the ongoing Phase IIb study and business development activities, with the ultimate goal of finding a suitable partner for the late-stage development of RV001.
Valuation: SEK885.1m or SEK46.5/share
We have increased our valuation of RhoVac to SEK885.1m or SEK46.5/share from SEK708m or SEK37.2/share previously. This was mainly due to rolling our model forward and a higher probability of success (15% vs 10% previously), which we have increased as the rights issue has removed the previous funding risk for the Phase IIb trial. The enrolment of the first patient to the Phase IIb study and Phase I/II data publication in a peer-reviewed article are some of the potential R&D newsflow in the near term. According to our model, a successful Phase IIb outcome would result in an increase in RhoVac’s rNPV to SEK2.0bn or SEK107.1/sh.
Business description
RhoVac is an immunotherapy company listed on the Spotlight stock market in Sweden, with a 100%-owned subsidiary in Denmark. It is developing a peptide-based immunotherapy, RV001, which aims to train the immune system to specifically target cancer cells with metastatic potential. This is a novel approach that could have utility across a range of cancer settings.
R&D update
Phase I/II final follow-up
On 4 July 2019, RhoVac released long-term follow-up results of the Phase I/II trial with RV001. The 22 prostate cancer patients who were enrolled to the trial received 11 subcutaneous injections over 30 weeks. The primary objective was to evaluate safety and tolerability, while the secondary objective looked at immune response. The patients were monitored for 12 months. Treatment-related reactions and immunological response (measured by IFNγ ELIspot analysis) were assessed at three, six, nine and 12 months (positive findings after the three- and six-month follow-ups had already been reported). The July announcement was the final update on patient follow-up in the Phase I/II trial. In summary:
All 22 patients completed the follow-up and no treatment-related adverse reactions were observed. In total 21 patients were evaluable for immune activation.
As reported previously, 18 out of 21 patients (86%) showed significant treatment-related immunological response after treatment with RV001. This significant response was maintained in the same 18 patients at three-, six- and nine-month follow-up. At the 12-month point, 17 patients still showed significant immunological response.
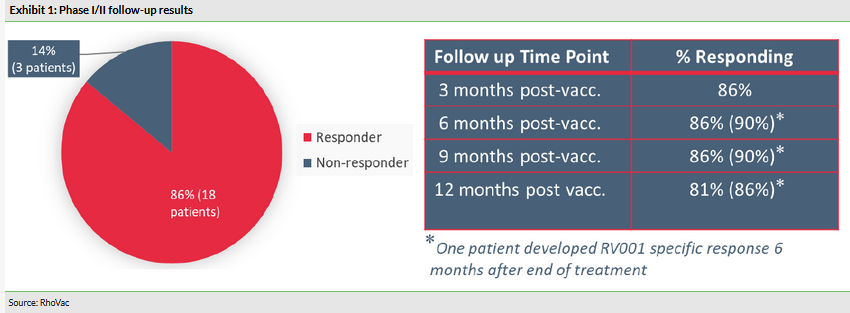
We view the Phase I/II results as positive, as RV001 looks to be a very safe treatment and elicits a long-lasting immune response in a large majority of patients. So, RV001 appears to do what it was designed for. The question of whether immune activation will translate into clinical efficacy will be addressed in a controlled trial that is already underway. RhoVac should publish the Phase I/II results in a peer-reviewed article in the near future.
Phase IIb first patient to be enrolled imminently
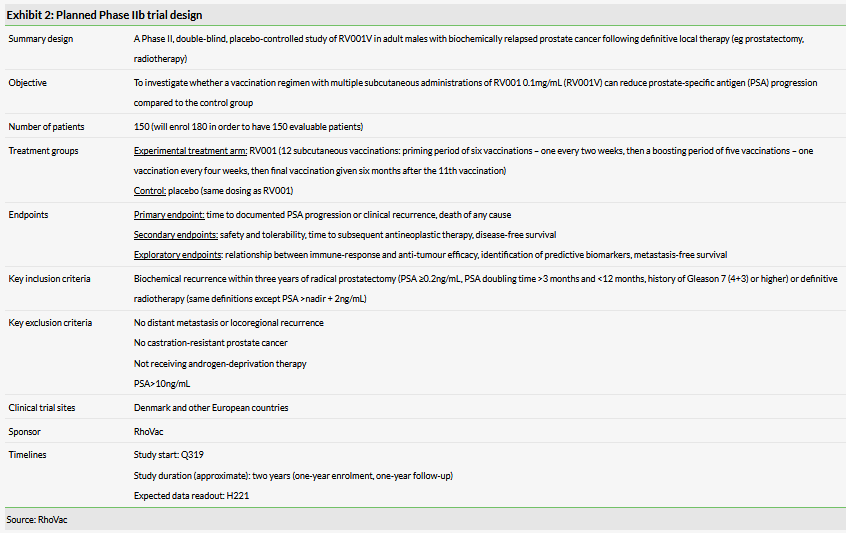
Following publication of positive initial Phase I/II data, RhoVac has designed a larger Phase IIb study to explore efficacy in the same patient group (Exhibit 2). The trial is expected to enrol the first patient any time now. Most of the study centres are in Europe, but RhoVac has confirmed it plans to open several US centres. The therapy will be administered as 12 subcutaneous injections over several months and final data are expected in H221. Exhibit 2 summarises the trial design.
New CEO
On the corporate side of the business, RhoVac announced on 2 September 2019 that Anders Månsson had been appointed as the new CEO. Former CEO Anders Ljungqvist will continue working as chief operating officer. Mr Månsson joined RhoVac in May 2019 as deputy CEO and chief business officer. He has extensive experience in the pharmaceutical and biotech industries and has worked in senior positions in major pharmaceutical companies in Sweden, Denmark, the UK and Switzerland. His focus was on sales and marketing, as well as on business development including distribution, licence agreements, divestments and acquisition agreements worth over several billion SEK. In recent years, he also held a number of board positions in biotech/life science companies and was the CEO of a stem cell company in Lund.
We believe Mr Månsson’s expertise is a good fit strategically because RhoVac’s areas of focus over the next three years will be executing the ongoing Phase IIb study and business development activities, with the ultimate goal of finding a suitable partner for the late-stage development and global launch of RV001. The fact that former CEO and co-founder Anders Ljungqvist is staying on as COO is also very positive as RhoVac will maintain the expertise it has accumulated in RV001 technology.
Significant EU grant received
On 22 August 2019, RhoVac announced that the EU Commission had granted it €2.5m from the EU framework programme Horizon 2020. Over the next 36 months, RhoVac will receive grant payments to support the Phase IIb trial with RV001 in prostate cancer and RhoVac’s business development activities. This is substantial support for RhoVac, bearing in mind that the recently completed rights issue amounted to SEK181m, which is expected to be sufficient to fund budgeted activities until 2022. In addition to the financial benefit, we view the grant as a form of external validation, since proposed business plans and technology are subject to scrutiny by the EU Commission.
Financials and valuation
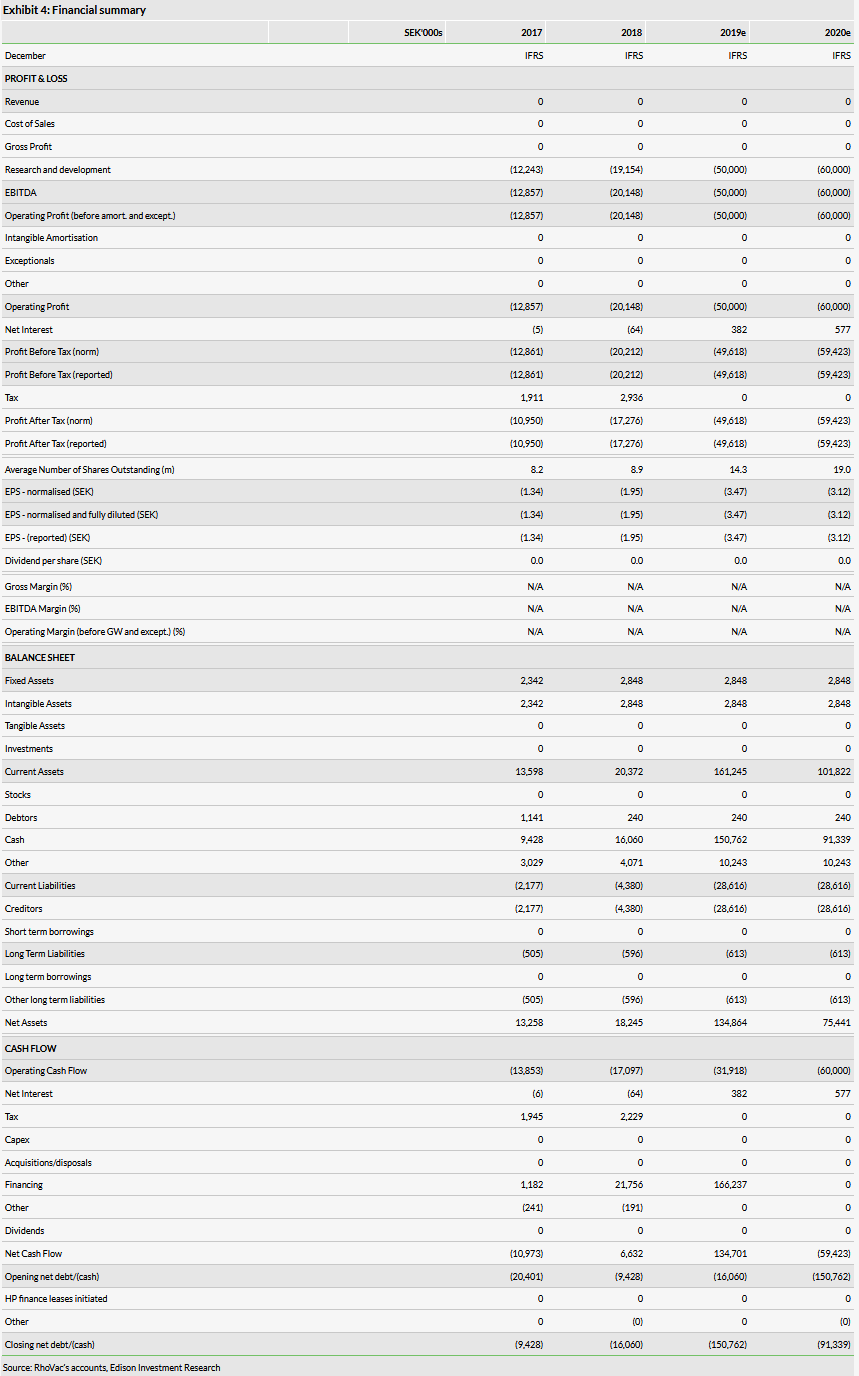
RhoVac reports no income, while the operating spend was SEK30.1m in H119, up from SEK7.9m in H118, mainly because of the preparations and initiation of the Phase IIb study. We already expected operating expenditure to increase as the Phase IIb accelerates after the right issue. Our previous operating loss estimates were SEK37m and SEK50m for 2019 and 2020 respectively, which we now revise to SEK50m and SEK60m.
RhoVac received SEK5.1m in tax credits in H119. In June 2019, it completed a rights issue raising a total of SEK180.9m gross (SEK166.2m net). In total, 9,523,551 shares were issued (a 100% increase in the number of shares outstanding) at a price of SEK19/share (vs SEK34.9/share on the day before the announcement). The rights issue was guaranteed by commitments from a group of investors. The newly raised funds should be sufficient to fund RhoVac’s operations for the next three years. With its H119 report, RhoVac reported SEK68.8m in cash and no debt. SEK105.7m was booked in receivables, as the rights issue was completed in July 2019.
We have increased our valuation of RhoVac to SEK885.1m or SEK46.5/share from SEK708m or SEK37.2/share previously. This was mainly due to rolling our model forward and increasing the success probability to 15% from 10%. As we discussed in our initiation report, we had assumed a somewhat more conservative success probability prior to the rights issue, as the project required substantial funding commitments. Now the rights issue has been successfully resolved, funding risk is lower and we increased the success probability to our standard 15% (Phase II to approval for oncology projects). We maintain all other assumptions in our risk-adjusted NPV model as published in our recent initiation report. Our valuation is based on RV001 in prostate cancer only, specifically in patients with biochemical recurrence following radical prostatectomy or radiotherapy.
According to our model, a successful Phase IIb outcome would result in RhoVac’s rNPV increasing to SEK2.04bn or SEK107.1/share (not including the net cash estimate). This would include setting the probability of success at 40% as a Phase III-ready asset and changing the date of the valuation to the start of 2022 but leaving all other inputs unchanged.