NovaBay’s (NBY) cash runway should now extend into 2014 (previously H213), with recent and imminent injections of cash totalling $5.6m. Galderma’s initiation of a Phase IIb trial with NovaBay’s NVC-422 for impetigo prompted a $2.6m milestone payment, while $1.5m was received from the expansion of a marketing agreement with Naqu Area Pioneer Pharma in South-East Asia for NeutroPhase (a further $1.5m is due by 31 October 2012). NVC-422, a topical anti-infective, is now being studied in three Phase IIb trials for significant unmet needs and results through 2013 offer re-rating potential and further financing/partnering opportunities.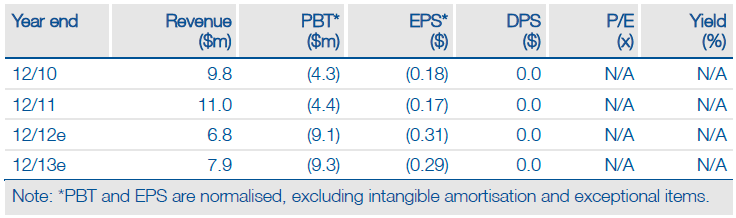
Impetigo trial underway
Galderma has started patient enrolment into a Phase IIb study of NVC-422 for mildto- moderate impetigo, triggering a $2.6m milestone payment to Novabay. The trial is expected to enroll 300 patients and should report top-line data by Q313. Positive results would trigger a further single-digit, million-dollar milestone (we estimate $3.5m) and, subject to a confirmatory Phase III, FDA approval/launch is possible by H116.
New strategic partner
NovaBay has expanded its existing relationship with China-based Pioneer Pharma to market NeutroPhase (skin/wound cleanser product) throughout South-East Asia, delivering $3m upfront in cash/equity investment. Pioneer now holds a 5.3% stake in NovaBay, which could increase to 13.5% if all options and warrants are exercised (based on current 28.9m shares outstanding). Cash runway extension The $5.6m funds from Galderma and Pioneer now extend NovaBay’s cash runway into 2014, while a potential extra $3m from Pioneer in 2013 would stretch it further. This is significant as it ensures NovaBay reaches a number of potential catalysts in 2013 with results from three Phase IIb trials of NVC-422.
Valuation: Increased to $60m
We have increased our valuation of NovaBay to $60m (previously $53m), or $1.96 per share, based on a sum-of-the-parts DCF valuation. The increase is due to a stronger
net cash position (estimated at $13.1m) and an upgrade in the probability of success for NVC-422 in impetigo to 50% (previously 45%). This represents clear upside to
NovaBay’s current market capitalisation of $37m and $1.27 share price.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
NovaBay Pharmaceuticals' New Strategic Parnter Extends Cash Runway
Published 10/21/2012, 07:26 AM
Updated 07/09/2023, 06:31 AM
NovaBay Pharmaceuticals' New Strategic Parnter Extends Cash Runway
Cash runway extension
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.