In February 2019, NeuroVive (ST:NVP) completed a rights issue, which was followed by a private placement. These brought in a total of SEK108.1m (net estimated), which should be sufficient to 2020. In its recent 2018 annual report, NeuroVive provided a detailed update on its R&D activities and outlined the goals for 2019 achievable with the new funding. Potential near-term share price catalysts include KL1333 Phase Ib initial results, non-dilutive financing and the start of the NeuroSTAT Phase II clinical trial, and an out-licensing of NV556. Our updated valuation is SEK1.51bn or SEK8.1/share.
KL1333 Phase Ia/b trial top-line data in 2019
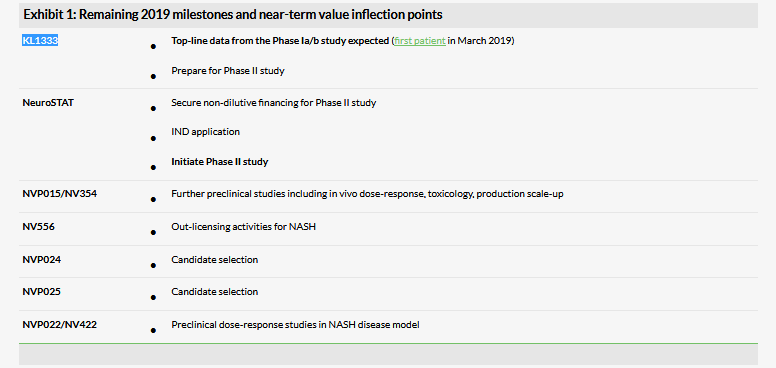
The newly raised funds will be used to advance NeuroVive’s clinical and preclinical programmes, with one of the key trials being the Phase Ia/b study testing KL1333 in development for mitochondrial diseases, such as MELAS, PEO, KSS and Pearson’s syndrome. KL1333, a small molecule NAD+ modulator, was tested in healthy volunteers in a Phase I trial (single ascending dose) by the licensor Yungjin Pharm in South Korea, which found the compound safe, with a favourable PK profile. NeuroVive’s own Phase I trial will include multiple ascending doses in healthy volunteers and patients. We therefore believe the likelihood of confirming the safety profile is high, but additional interesting early efficacy insights could be obtained from patient data. The trial has already enrolled the first healthy volunteer. Initial data from healthy volunteers are expected in H219.
NeuroSTAT, NV556 and NV354 also in focus in FY19
NeuroVive also plans to initiate the proof-of-concept Phase II trial with NeuroSTAT in traumatic brain injury, where there is no specific, approved therapeutic treatment. Additional funding will be required to complete the trial, which management indicated could be carried out via non-dilutive funding or a partnership. Out-licensing preclinical assets in the non-core portfolio, especially NV556 (NASH), is another potential catalyst and a source of cash. NV354 (selected compound from the NVP015 programme) is one of the preclinical projects also gaining pace and could enter the clinic in 2020. Our last outlook report describes the ongoing R&D programmes in detail.
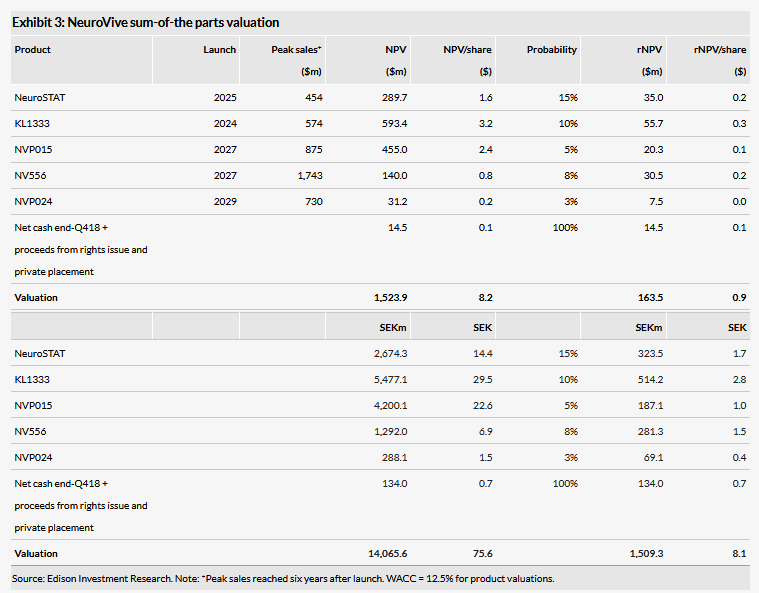
Valuation: SEK1.51bn or SEK8.1/share
Our updated, risk-adjusted NPV valuation of NeuroVive is SEK1.51bn or SEK8.1/share compared to SEK1.51bn or SEK9.2/share previously due to an increased cash position, revision of the operating and rNPV models, and rolling the model forward. The slight decrease in valuation per share is mostly technical adjustment after the increased number of shares.
Business description
NeuroVive Pharmaceutical is a Swedish biopharmaceutical company with deep expertise in mitochondrial medicine. It has a diversified portfolio in terms of indications and employs a dual strategy: it develops a core portfolio of assets for orphan diseases and seeks to out-license proprietary products for non-orphan indications. NeuroSTAT (neurotrauma, Phase IIb ready) and KL1333 (genetic mitochondrial diseases) are the most advanced assets.
Financials and valuation
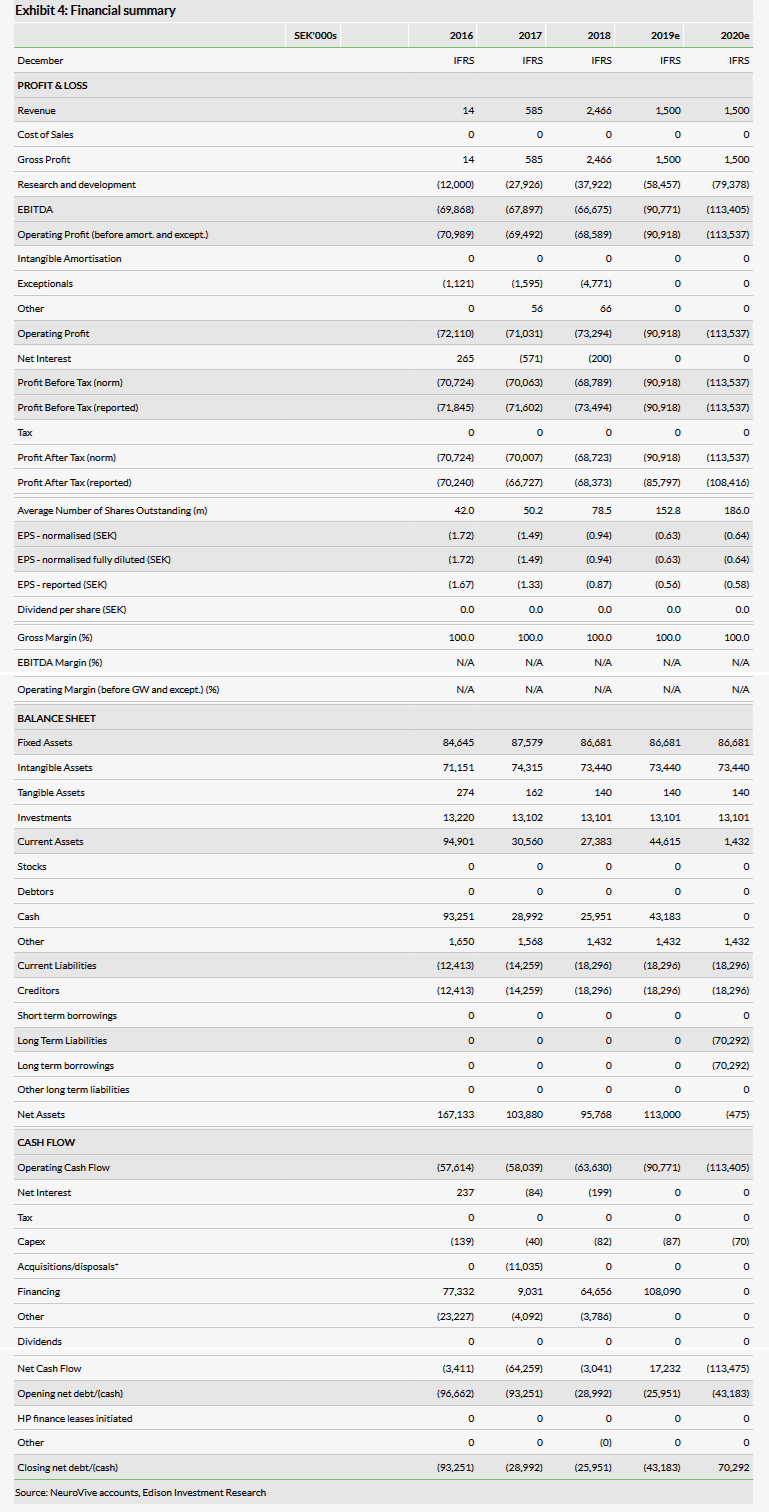
The rights issue raised a total of SEK99m gross or an estimated net SEK81m (guarantee commitment fee was SEK9.1m). A few weeks after the share issue, NeuroVive announced a private placement, which brought in an additional SEK28.2m gross. The total number of shares outstanding increased from 91.7m to 186.0m after the rights issue and the private placement. Previously we have already included the guaranteed amount of SEK93m in our model, while the total newly raised funds amount to SEK108.1m (net of fees estimate), which we include in our model now. This and the last reported end-Q418 cash of SEK26.0m should be sufficient to fund operations into 2020, according to the company, which is also in line with our model. This includes several R&D events as discussed, most notably expected top-line data from the Phase Ia/b trial with KL1333 and the initiation of the Phase II study with NeuroSTAT.
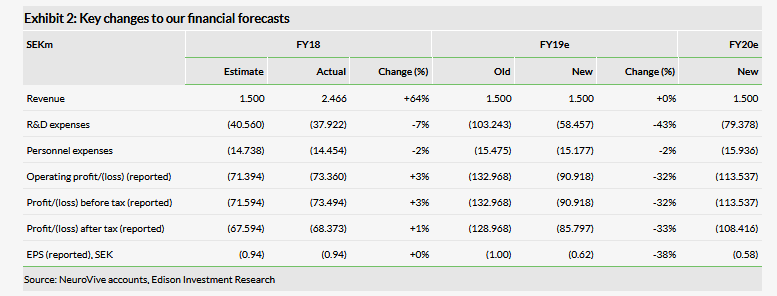
In 2018, total R&D-related expenses were SEK37.9m versus SEK27.9m, mainly due to a combination of more intensive R&D, but also the change in accounting approach from capitalising R&D costs to expensing in April 2017. Although NeuroVive’s FY18 results were largely in line with our expectations, we reviewed our R&D spend projections after the company published its 2018 annual report, in which the 2019 goals were listed. We have made no changes to the lead projects NeuroSTAT and KL1333, as these trials are progressing with the new funding. The main revisions to our R&D model include moving the expected licensing deal for NV556 from 2018 to 2019 and the initiation of the Phase I trial with compounds from the NVP015 programme from 2018 to 2020 (we note that a subset of NVP015 compounds was out-licensed to BridgeBio Pharma in June 2018, as discussed in our outlook report). These were the main reasons for the downward revision of R&D costs in FY19.
Our updated, risk-adjusted NPV valuation of NeuroVive is SEK1.51bn or SEK8.1/share compared to SEK1.51bn or SEK9.2/share previously due to the increased cash position, revision of the operating and rNPV models, and rolling the model forward. We maintain all other R&D assumptions as described in our initiation report and last outlook note. As previously, in our valuation we include clinical-stage NeuroSTAT (TBI) and KL1333 (genetic mitochondrial disorders), and the advanced preclinical products. We continue to exclude NVP025 (mitochondrial myopathy) and NVP022 (NASH) for the time being, as both are at an early stage.