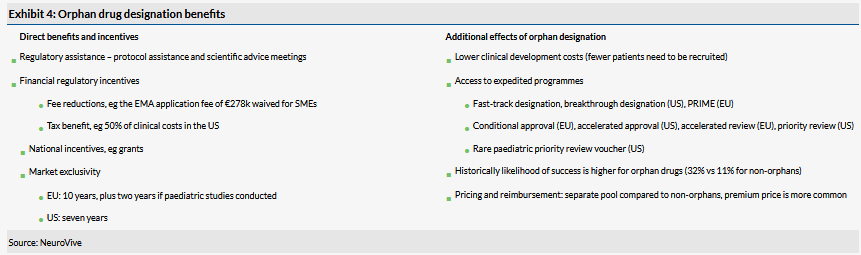
On 9 October 2019, NeuroVive Pharmaceutical AB (ST:NVP) held its capital markets day. Management presented its strategic focus on the company's primary mitochondrial disease programmes, high unmet medical need in this area and advantages of the orphan drug development compared to drugs for common diseases. As expected, no major new details were disclosed. We note an interesting presentation on regulatory perspectives on orphan drug development by NeuroVive’s clinical and regulatory affairs director, Matilda Hugerth. It is not that often investors have straightforward access to expert knowledge about such a specialised topic presented in a clear format. Our valuation is unchanged at SEK1.63bn or SEK8.8/share.
KL1333 and NV354 lead assets in core portfolio
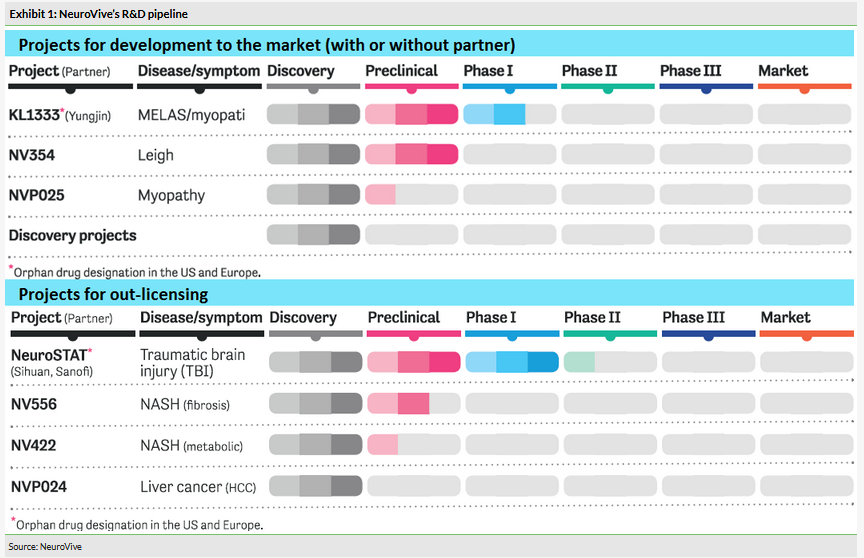
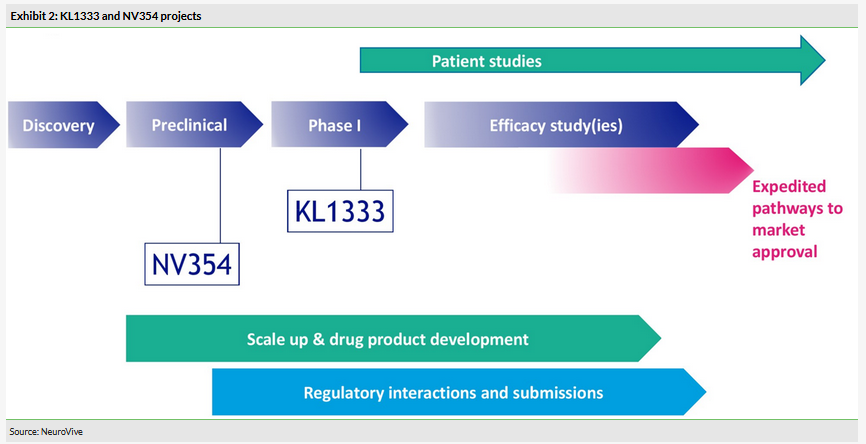
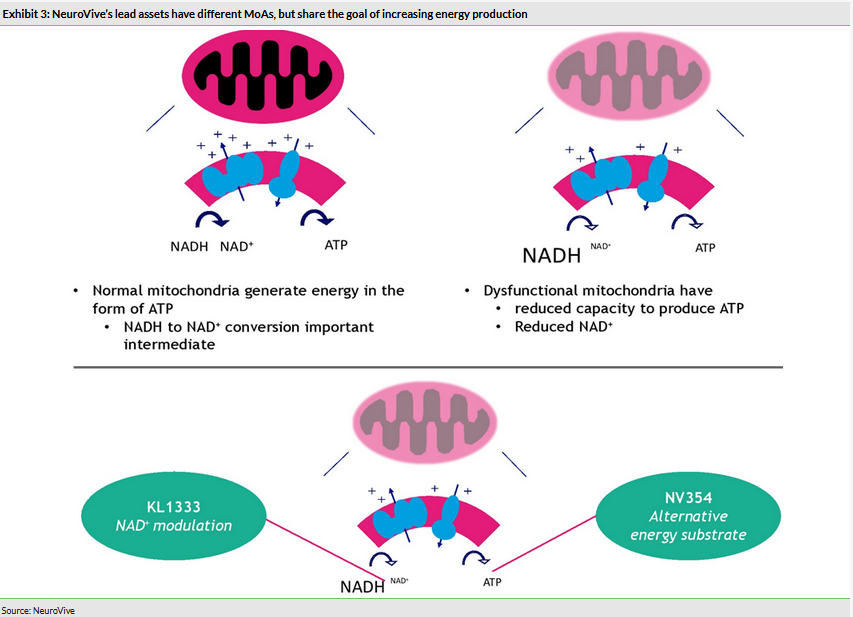
KL1333, a small molecule NAD+ modulator used to restore intracellular energy balance, is in the multiple ascending dose (MAD) part of the Phase Ia/b trial. The single ascending dose (SAD) part of the trial delivered positive PK and safety data. KL1333 is being developed for primary mitochondrial disease, for example due to an m.3243 A>G mutation (eg MELAS, MIDD, PEO). The MAD part will also recruit mitochondrial disease patients (expected in H120). This will be the first time KL1333 is tested in patients and could potentially deliver interesting initial efficacy signs. If results are positive, the Phase II trial could start as soon as 2020. NV354, a succinate prodrug targeting complex I deficiency (such as Leigh syndrome and LHON), is the second lead drug candidate in the core portfolio targeting mitochondrial disease. The mechanism of action (MoA) is different to KL1333, but has the same goal of increasing the production of cellular energy (Exhibit 3). IND-enabling studies are ongoing and the Phase I study could start in 2020.
NeuroSTAT ready for Phase II development
NeuroSTAT, an innovative formulation of ciclosporin, is the most advanced asset in the portfolio for out-licensing and partnering and is positioned for the treatment of traumatic brain injury, where there is no neuroprotective treatment available yet. NeuroSTAT has accumulated some initial efficacy data and has received IND approval from the FDA. NeuroVive indicated that while promising, NeuroSTAT will require too much resource for NeuroVive to take to the market and hence it is focusing on finding partners that can finance the Phase II trial.
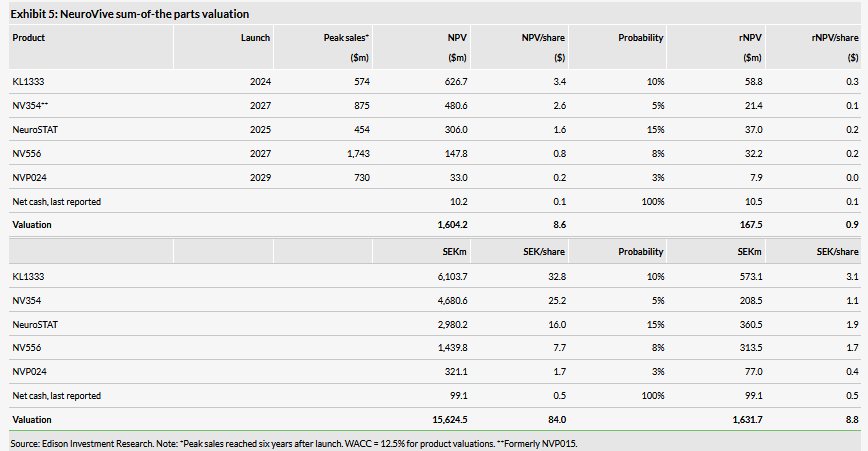
Valuation: SEK1.63bn or SEK8.8/share
We keep our valuation unchanged at SEK1.63bn or SEK8.8/sh. Potential near-term milestones include initial results from KL1333 Phase Ia/b, a non-dilutive financing solution for NeuroSTAT Phase II trial and NV354 entering clinical development. More detail on the projects are given in our recent update report.
Business description
NeuroVive Pharmaceutical is a Swedish biopharmaceutical company with deep expertise in mitochondrial medicine. It has a diversified portfolio in terms of indications and employs a dual strategy: it develops a core portfolio of assets for orphan diseases and seeks to out-license proprietary products for non-orphan indications. KL1333, NV354 (mitochondrial diseases) and NeuroSTAT (neurotrauma) are the most advanced assets.
Capital markets day take aways (exhibits)