Mologen (DE:MGNK) has announced that its pivotal Phase III IMPALA trial testing lefitolimod as a maintenance therapy in patients with metastatic colorectal cancer (mCRC) has missed its primary endpoint of overall survival (OS). As a result, we have removed from our valuation lefitolimod’s prospects as a monotherapy in mCRC and small cell lung cancer (SCLC) (previously 72% of our valuation). MOLOGEN will focus on the development of lefitolimod and EnanDIM in combination with other therapies. Recently announced restructuring measures should reduce monthly cash burn to €0.8m from the current rate of €1.4m. We note gross cash as of 30 June 2019 was €6.0m, which should enable funding into Q419. We value MOLOGEN at €50.4m (€4.1/share) vs €169m (€18.2/share) previously.
IMPALA Phase III trial primary endpoint not met
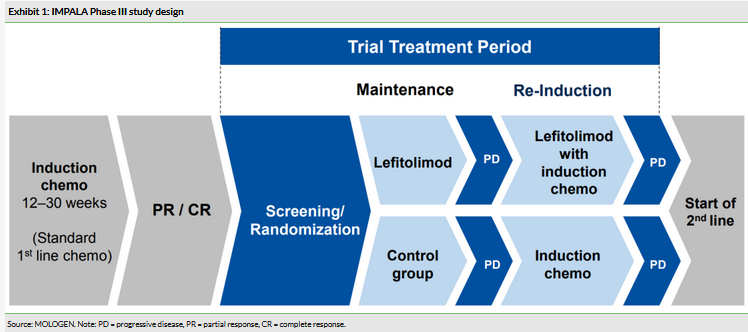
549 patients were enrolled in the two-arm, randomised pivotal Phase III trial for the maintenance treatment of mCRC patients. The primary endpoint of a statistically significant increase in overall survival was not met. Patients in the lefitolimod group demonstrated a median OS of 22.0 months compared with 21.9 months in the control group with a hazard ratio of 1.12 (95% CI: 0.91–1.38). MOLOGEN now plans to focus on combinations for lefitolimod and its early-stage EnanDIM assets. An ongoing trial is testing Yervoy (BMY – CTLA-4 antibody) and lefitolimod in patients with solid tumours, while the TITAN study (funded by Gilead (NASDAQ:GILD)) in HIV patients testing lefitolimod in combination with novel antibodies is expected to begin shortly. MOLOGEN also expects to start an anti-PD1 combination study soon with its partner, Oncologie, in the near term if funding is achieved.
Financials: Cash reach into Q419
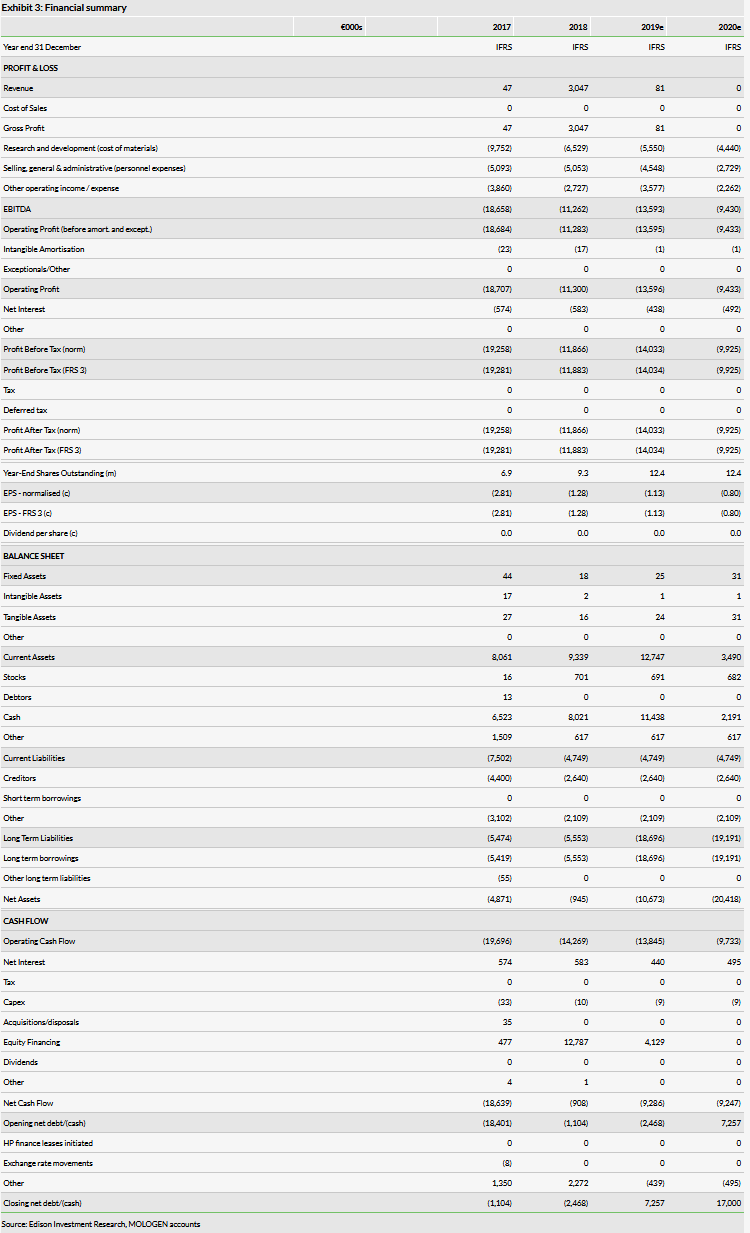
The net loss increased to €8.0m versus €4.8m in H118, resulting in gross cash at 30 June of €6.0m. In addition, as at the mid-year, MOLOGEN had €6.3m in long-term convertible bond liabilities. At the recent AGM, shareholders agreed a new authorised and conditional capital facility in addition to authorisation to issue new convertible bonds and/or option bonds. We model funding (as illustrative debt) of €10m by the year end to fund operations into 2020.
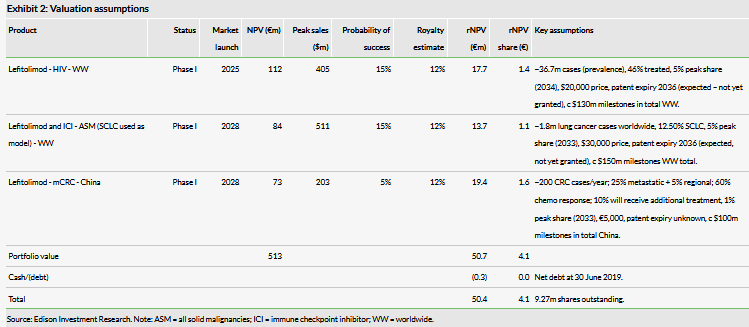
Valuation: €50.4m (€4.1/share)
We value MOLOGEN at €50.4m (€4.1/share) vs €169m (€18.2/share) previously, updated for the failure of the IMPALA trial. As a result of this data we have removed lefitolimod’s potential as a monotherapy in CRC and SCLC. In addition, we have rolled forward our model, and updated for FX and the increased number of shares.
Business description
MOLOGEN is a German biopharmaceutical company developing novel biopharmaceuticals. The company’s lead product, lefitolimod (TLR9 agonist), is being evaluated in HIV and a combination trial in advanced solid malignancies.
IMPALA data signal the end of monotherapy strategy
IMPALA, a 549-patient (enrolled), two-arm, non-blinded, randomised pivotal Phase III trial for the maintenance treatment of mCRC patients did not meet is primary endpoint of generating a statistically significant increase in overall survival. The trial was conducted across 120 centres in eight European countries. Patients in the lefitolimod group demonstrated a median OS of 22.0 months compared with 21.9 months in the control group with a hazard ratio of 1.12 (p=0.2765; 95% CI: 0.91–1.38). In predefined subgroups, lefitolimod demonstrated no benefit while progression free survival (PFS) was superior in the control arm. No new safety signals were observed. MOLOGEN notes that it no longer sees a future for lefitolimod as a monotherapy and all future trials will be based around combination therapies.
MOLOGEN now plans to focus on combinations for lefitolimod and its early stage EnanDIM assets. A trial is ongoing testing Yervoy (BMY – CTLA-4 antibody) and lefitolimod in patients with solid tumours. At SITC 2018, first clinical data were presented on the combination of the immune checkpoint inhibitor Yervoy and lefitolimod in patients with solid tumours. To date, 19 patients have been enrolled and no dose-limiting toxicities were encountered at any dose level. The combination was generally well tolerated and safe. Two patients experienced stable disease for 45 weeks (primary peritoneal carcinoma) and 24 weeks (high-grade pancreatic neuroendocrine tumour), respectively (NCT02668770).
Together with its partner, Oncologie, MOLOGEN plans to start an anti-PD1 combination study soon, in the near term if funding is achieved. In addition, two further combination studies in solid cancers are in advanced stages of planning and could start soon if funding is raised.
In HIV, the TITAN study (funded by Gilead (NASDAQ:GILD)) is expected to begin shortly. The trial will test lefitolimod with innovative antibodies developed by Rockefeller University. As with the previous TEACH study, the trial will be led by Aarhus University Hospital Denmark. MOLOGEN also notes that additional late-stage conversations are ongoing with renowned US centres to start further trials.
For its next generation TLR9 assets (EnanDIM), MOLOGEN notes that preclinical development of the first product candidate continues and forecasts that it should be ready for the clinic by year end.
H119 results: MOLOGEN’s cash needs are pressing
Based on MOLOGEN’s current cash burn, we forecast a cash reach into Q419 and model funding (as illustrative debt) of €10m by the year end to fund operations into 2020. At the AGM on 29 August, shareholders agreed a new authorised and conditional capital facility in addition to authorisation to issue new convertible bonds and/or option bonds. We now anticipate that management’s main focus in the autumn will be on achieving the required funding to secure MOLOGEN’s future clinical development plans. Gross cash at 30 June was €6.0m, boosted by the €4.2m capital raise in April 2019.
Following the failure of the IMPALA Phase III trial, MOLOGEN has announced a new strategic programme including restructuring measures. This includes a reduction of staff to around one-third of the current number, with a final employee count of 15 (47 employees as of 30 June 2019). Additionally, it has withdrawn from two locations and will now wholly operate from one site. As a result of these changes, management expects to bring its monthly cash burn down to €0.8m from the current rate of €1.4m.
Revenue in H119 decreased to €81,000 (vs €3m in H118) as a result of the one-off Oncologie payment in H118. SG&A expenses decreased slightly year-on-year to €3.2m (vs €3.6m in H118) and €2.6m (vs €2.7m in H118) respectively. The net loss increased to €8.0m versus €4.8m in H118. We forecast a decrease in our FY19 net loss (to €14.0m from €17.0m previously) as we have lowered our assumed R&D (to €5.6m from €6.9m previously) and SG&A (to €4.5m from €5.5m previously) expenses as a result of the aforementioned restructuring plans. In FY20, we forecast a significant reduction in costs as the restructuring plans take complete effect.
MOLOGEN currently has €6.3m in non-current liabilities as a result of convertible bonds issued since 2016. In January 2019, the company issued a convertible bond with a nominal value of €2.7m, a term of eight years and fixed interest rate of 6.0%. In September 2018, MOLOGEN issued a €2m convertible bond to Oncologie without subscription rights. In November 2016 (2016/2024 bond: €2.54m) and in January 2017 (2017/2025 bond: €4.99m), two convertible bonds were placed. Terms of these were renegotiated at the start of 2019. We have modelled €10m of illustrative debt in 2019 to enable funding into FY20.
Valuation: €50.4m (€4.1/share)
We value MOLOGEN at €50.4m (€4.1/share) vs €169m (€18.2/share) previously, updated for the failure of the IMPALA Phase III trial. As a result of this data we have removed lefitolimod’s potential as a monotherapy in CRC and SCLC. Additionally, we have rolled forward our model, and updated for FX and the increased number of shares following the capital raise in April. The valuation is based on a risk-adjusted, sum-of-the-parts DCF model, applying a standard 12.5% discount rate and net debt of €0.3m.