Medigene’s long search for a development partner for its Phase II breast cancer candidate EndoTAG-1 has reached a significant milestone with SynCore Biotechnology acquiring development and commercialisation rights in Asia, Australia and New Zealand. Medigene retains rights in the US and Europe to the paclitaxel-based drug and continues to seek partners for these key territories. Medigene now expects an NDA filing in 2018, so we assume a potential launch in 2019, a two-year delay to our prior estimate, which reduces our valuation by €9m to €87m.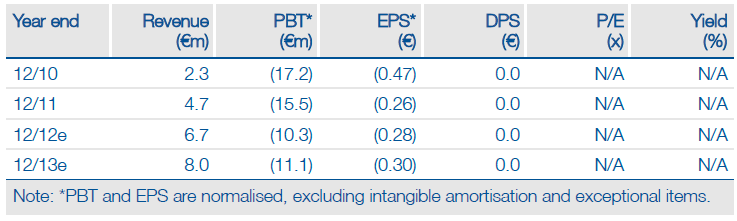
Wider Relationship
SynCore, a subsidiary of Sinphar Pharmaceutical and already a partner of Medigene’s in Taiwan for the commercialisation of genital warts ointment Veregen, will take over all development and commercialisation duties for EndoTAG-1 in Asia, Australia and NZ. Specific financial terms of the deal were not disclosed. Medigene will receive a modest upfront fee (we assume <€1m as the deal does not affect Medigene’s financial guidance for 2012), and is eligible for development/approval milestones and royalties.
50% Cost Of Pivotal Trial Covered
Medigene is now planning a global Phase III pivotal trial for EndoTAG-1 in 400 women with triple-negative breast cancer (TNBC), 50% of whom will be recruited in Asia. SynCore will therefore cover half of the estimated €20m cost of the trial (we assume an average €50k per patient cost for a Phase III cancer study).
Further Partnership Potential
With Medigene making no changes to its financial guidance for 2012 and still expecting existing cash to extend beyond 2013, we assume the company continues to seek EU/US partners to fund the remaining 50% (€10m) of the pivotal study. The desire to secure further partners for EndoTAG-1 and the need to scale up the manufacturing process help to explain the six-year gap to NDA filing. Ultimately, Medigene remains focused on the development of anti-rheumatic agent Rhudex.
Valuation: Reduced By €9m To €87m
The longer than expected development timeline for EndoTAG-1 reduces our valuation of Medigene by €9m to €87m. This includes our net cash estimate of €26m at the end of June 2012 and shows clear upside to the company’s €44m market capitalisation. We have not made any changes to our financial model, which currently predicts that Medigene is likely to have a funding requirement in H214.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Medigene's Asia Deal
Ally In Asia For EndoTAG
Latest comments
Loading next article…
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.