InMed Pharmaceuticals Inc (TO:IN) recently reported results for the third quarter of FY19 and is on track to be in the clinic to treat epidermolysis bullosa (EB) in the second half of this year (consistent with prior guidance for a CTA filing in H219 with entrance into clinic by year-end 2019). In March, it announced that instead of developing a combination cannabinoid product for EB, it would go with a single agent (INM-755) as it discovered in its testing that by increasing the dose of one of the components, efficacy was the same as the combination. This greatly simplified the regulatory process as there are extra hurdles for a combination of two investigational drugs in one product.
Entering the clinic in the second half of 2019
InMed is on track for initiation of the Phase I study in healthy volunteers in H219. Importantly, the company has selected a contract manufacturing organization to produce drug product for the clinical trial, which provides further confidence in timelines for the trial. Enrolment is expected to occur quickly so results should be released sometime in the first half of 2020.
Biosynthesis development progressing
InMed completed the technology transfer to the National Research Council Canada (NRC) to support scale-up activities. The precursors were successfully converted into a specific cannabinoid using the correct construct in E. coli. Additionally, ‘downstream process’ (DSP) activities related to purification process development have begun.
Significant interest in biosynthesis of cannabinoids
Interest in the biosynthesis sector continues to grow with two partnerships recently announced. In March, Amyris announced a $300m agreement to produce cannabinoids in yeast with LAVVAN. In April, Zenabis Global announced a three-year supply agreement with Farmako for cannabidiol (CBD) biosynthesized in tequila bacteria, with a stated goal to begin supplying it in Q419, though this may be aggressive given the early stage nature of Farmako’s process.
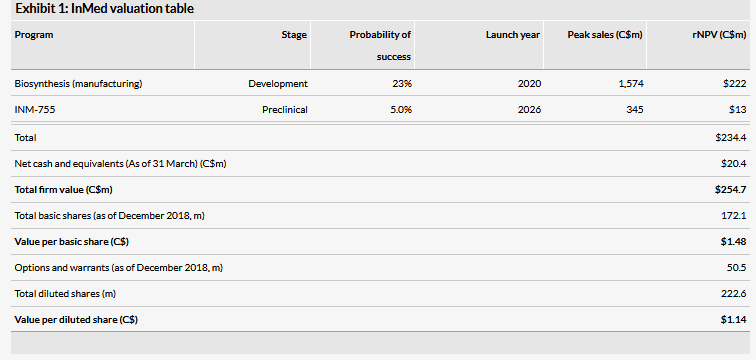
Valuation: C$255m or C$1.48 per basic share
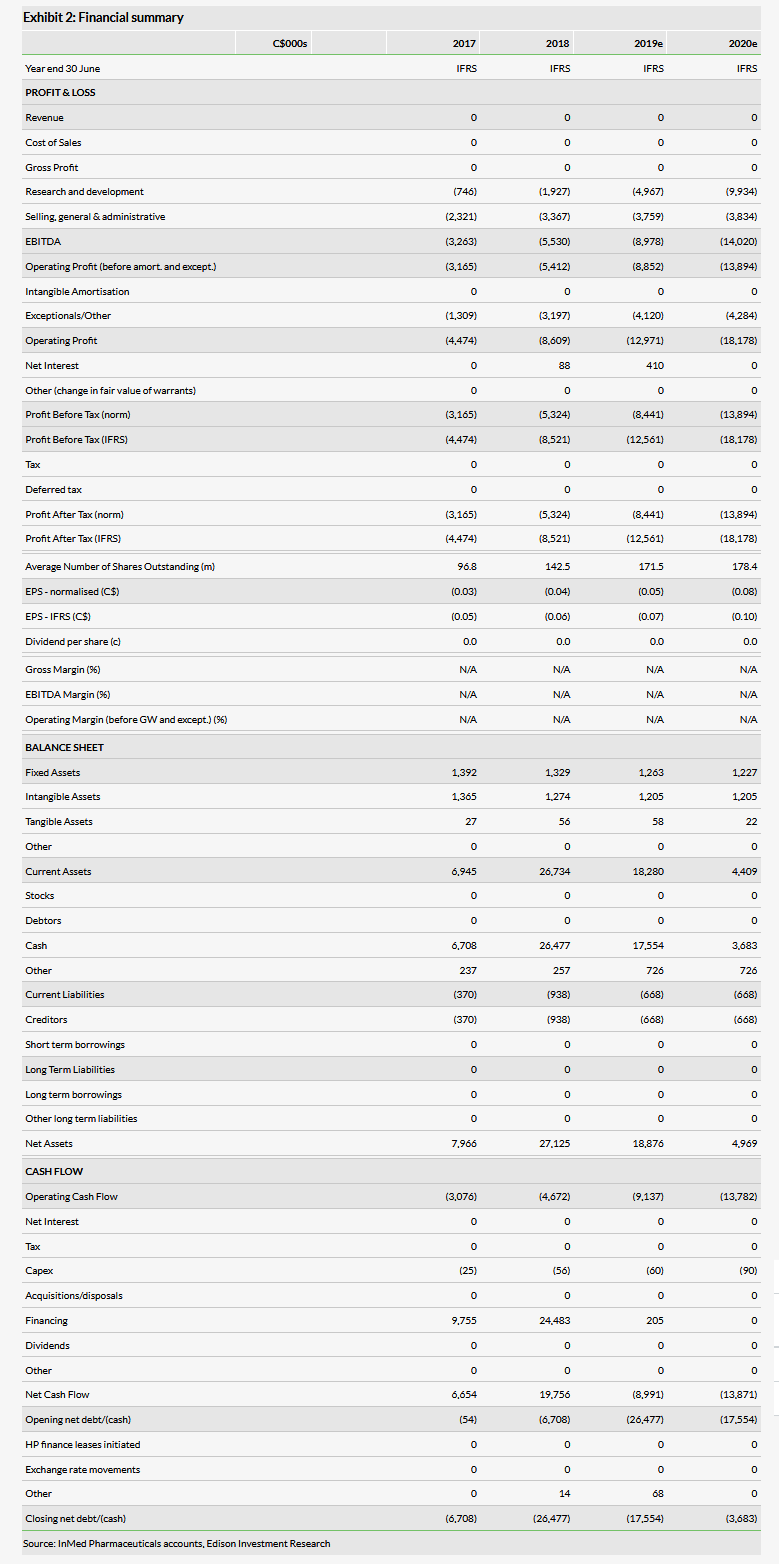
We have slightly adjusted our valuation to C$255m or C$1.48 per basic share (C$1.14 per diluted share), from C$257m or C$1.51 per basic share (C$1.16 per diluted share), principally due to lower net cash. InMed had C$20.4m in cash at 31 March and we believe this provides a runway through FY20.
Business description
InMed Pharmaceuticals is a Canada-based biopharmaceutical company focused on manufacturing and developing cannabinoids. Its biosynthesis platform may be able to produce cannabinoids for less cost and with improved purity compared to currently used methods. The company is also developing a proprietary pipeline, including INM-755 for epidermolysis bullosa, a serious, debilitating orphan indication.
An update on EB and biosynthesis
InMed continues to be on track for initiation of the Phase I study in healthy volunteers in H219. As a reminder, the company previously stated that it expects to enrol approximately 30 patients in total for the trial and for it to be a two-part study that will test two different drug concentrations in each part. The first cohort will evaluate the safety, tolerability and PK of INM-755 cream once daily for 14 days in healthy volunteers with normal, intact skin. In a second cohort of healthy volunteers, the company will test the local safety and tolerability of applying INM-755 cream to small wounds once daily for seven days (the small wounds will be created at the clinical site and will largely mimic the types of wounds typically seen in EB simplex patients).
Importantly InMed has already selected both the clinical research organization (CRO) to run the human clinical trials and the contract manufacturing organization that will produce the topical cream necessary for the Phase I trial, which provides us with additional confidence that the current timelines will be met. With regards to the timing of the Phase I trial itself, it will likely enrol quickly (the company expects the enrolment period to be ‘a matter of weeks’) so results should be released sometime in the first half of 2020.
With regards to biosynthesis, InMed previously announced in October that it had entered into a development agreement with the National Research Council of Canada (NRC), the primary research and technology organization of the Canadian government, for biofermentation development and scale-up processes at the NRC’s dedicated facility in Montreal. Technology transfer activities to the NRC began in late 2018 and have now been completed, including the transfer of the gene construct containing the coding sequence for the enzymes responsible for cannabinoid production. Fermentation scale-up activities have also begun.
Biosynthesis industry activity increasing
Additionally, there have been some big announcements related to cannabinoid biosynthesis lately, though it is unclear at what stage these companies are at in developing a fermentation process for cannabinoids. As a reminder, in September 2018, Ginkgo Bioworks announced a deal with Cronos Group in which Cronos will pay Ginkgo up to $122m to develop a biosynthesis process for eight target cannabinoids. Of this $22m will be to fund R&D and foundry expenses with the rest of the potential payout related to hitting milestones, namely achieving a production cost of less than $1,000 per kilogram at a scale of greater than 200 liters. It is unclear whether this is achievable due to the large amount of expensive inputs. In March 2019, Amyris announced a $300m agreement to produce cannabinoids (starting with CBD), also in yeast, with LAVVAN, a cannabis-focused company formed in the previous month with undisclosed backing or capitalization. Most of the agreement was backend loaded, but $10m was received within the first month and an additional $10–20m is expected by the end of 2020. In April, Zenabis Global announced a three-year supply agreement with Farmako for CBD biosynthesized in tequila bacteria, with a stated goal to begin supplying it in Q419. This goal may be aggressive as Farmako only moved into its own laboratory space in April. It is also unclear if any upfront funding was provided.
InMed has stated that it has been approached by potential partners but that it is going to wait until the process is more advanced (or ideally, finalized) in order to maximize its return.
Valuation
We have slightly adjusted our valuation to C$255m or C$1.48 per basic share (C$1.14 per diluted share), from C$257m or C$1.51 per basic share (C$1.16 per diluted share), principally due to lower net cash. We expect to re-adjust our valuation following advancement of INM-755 into the clinic and as the biosynthesis process approaches commercialization.
Financials
InMed reported an operating loss of C$3.6m in Q3 of FY19 (the quarter ending 31 March 2019), up from C$2.2m for the same period a year ago. R&D expenses were C$1.6m in Q319, up from C$0.6m in the same quarter last year. We have increased our R&D estimates by C$0.4m in FY19 and by C$0.9m in FY20 as the spending was a bit higher than we had expected.
The company had C$20.4m in cash, cash equivalents and short term investments at 31 March and had an operating cash burn of C$6.4m through the first three quarters of FY19. As we expect R&D expenditures to accelerate in the coming quarters, we believe its cash level provides a runway through FY20.