Epizyme, Inc. (NASDAQ:EPZM) announced that it has submitted a new drug application (NDA) for an accelerated approval of its lead pipeline candidate, tazemetostat, to the FDA. The NDA seeks a nod for the candidate to treat patients with metastatic/advanced epithelioid sarcoma (ES), who are not eligible for curative surgery.
Tazemetostat, a first-in-class EZH2 inhibitor, is being evaluated for both solid tumors and hematological malignancies as a monotherapy and combination therapy to treat relapsed and front-line disease.
Following a recently conducted pre-NDA meeting held with the FDA, Epizyme has filed the NDA for the given indication, which is the first of the two NDA submissions that has been planned by the company for 2019. This regulatory filing was based on the updated efficacy and safety data from the phase II study on tazemetostat, which enrolled 62 patients in the ES cohort. The company plans to present the data at the annual meeting of the American Society of Clinical Oncology (ASCO) to be held in 2019.
Meanwhile, Epizyme plans to begin a global, randomized study on tazemetostat to support a full approval for the ES indication during the second half of 2019. If approved, tazemetostat will be the first commercially available EZH2 inhibitor and the first treatment, specifically indicated for ES patients.
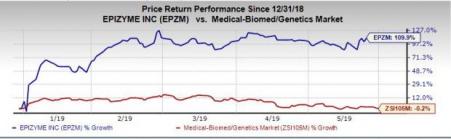
Shares of Epizyme were up almost 2.1% following this news on Thursday. In fact, the stock has skyrocketed 109.9% so far this year versus the industry’s decrease of 0.2%.
Notably, Epizyme plans to submit an NDA for the accelerated approval of tazemetostat pertaining to the patients with follicular lymphoma (FL), regardless of their EZH2 mutational status and also, who were previously treated with two or more systemic therapies. The NDA submission is planned for the fourth quarter of 2019.
The company is planning to initiate multiple clinical evaluations to extend the benefit of tazemetostat in the earlier treatment lines of FL and explore new combinations plus potential indications in both FL and solid tumors for its label expansion.
Epizyme also plans to discover the potential of tazemetostat in earlier lines of FL as combination therapy. The company is evaluating the opportunity to conduct a combination study that would compare the chemo-free combination of Roche’s (OTC:RHHBY) Rituxan and Celgene’s (NASDAQ:CELG) Revlimid with tazemetostat in comparison to the combo with placebo in patients with relapsed or refractory FL. Epizyme intends to provide an update on its combination study plans once the same is finalized.
Moreover, the company expects to initiate a combination probe on tazmetostat in mid-2019, testing patients with castration-resistant prostate cancer. The company also aims to begin investigations on small-cell lung cancer, triple-negative breast cancer and ovarian cancer in the second half of 2019.
Zacks Rank & Other Key Pick
Epizyme currently carries a Zacks Rank #2 (Buy). Another top-ranked stock in the healthcare sector is Acorda Therapeutics, Inc. (NASDAQ:ACOR) , which sports a Zacks Rank #1 (Strong Buy). You can seethe complete list of today’s Zacks #1 Rank stocks here.
Acorda’s loss per share estimates have been narrowed 6.5% for 2019 and 6.9% for 2020 over the past 60 days.
This Could Be the Fastest Way to Grow Wealth in 2019
Research indicates one sector is poised to deliver a crop of the best-performing stocks you'll find anywhere in the market. Breaking news in this space frequently creates quick double- and triple-digit profit opportunities.
These companies are changing the world – and owning their stocks could transform your portfolio in 2019 and beyond. Recent trades from this sector have generated +98%, +119% and +164% gains in as little as 1 month.
Click here to see these breakthrough stocks now >>
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
Epizyme, Inc. (EPZM): Free Stock Analysis Report
Celgene Corporation (CELG): Free Stock Analysis Report
Acorda Therapeutics, Inc. (ACOR): Free Stock Analysis Report
Original post
Zacks Investment Research
