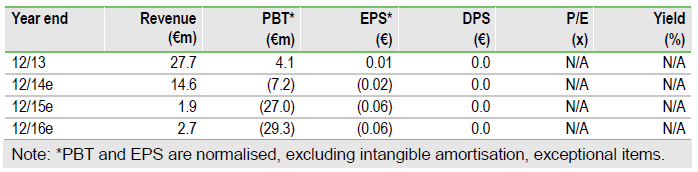
Focus remains on tozadenant
A Phase II study with nepicastat (SYN117) for cocaine dependence did not meet its primary efficacy endpoint. While disappointing for patients seeking treatment options for their addiction, from Biotie’s perspective we view this as a neutral event – the trial was fully-funded by the US NIH and we did not include nepicastat in our valuation model. We maintain our rNPV of Biotie’s product portfolio at €164m. Our focus is on the Phase III programme for tozadenant in Parkinson’s disease, due to start in H115, and the additional finance and/or partner required to fully fund the study.
Nepicastat was never a core product
Nepicastat was acquired from the purchase of Synosia in 2011, which had licensed the compound from Roche (SIX:ROG) in 2007. Clinical development, in cocaine dependence and a prior Phase II study in post-traumatic stress disorder (also missed primary endpoint in 2012), has been fully-funded by US government agencies, with minimal investment from Biotie (clinical material costs only). We have never included nepicastat in our valuation model. Cocaine dependence is a notoriously difficult-totreat disorder, and nepicastat failed to increase abstinence rates (measured in the last two weeks of the 11-week treatment period) in the 179-patient study.
Tozadenant development on track
Biotie (HEL:BTH1V) is focused on starting a Phase III clinical programme with tozadenant (A2a antagonist), in PD patients with motor fluctuations on levodopa, on track to start in H115. This is likely to require 900 patients and is supported by highly encouraging Phase IIb (n=420) data. With estimated FY14e cash of €33m, Biotie requires additional finance and/or a partner to ensure full-funding for the study, which we forecast at $85m.
To Read the Entire Report Please Click on the pdf File Below