We’ve been following AVEO Pharmaceuticals on Catalyst~Watch ahead of the AdCom vote today (May 2nd, 2013). The catalyst has been triggered – AVEO is down 48.3% at the time of writing.
Today, the FDA’s Oncologic Drugs Advisory Committee meeting voted 13-1 against the question on whether AVEO’s Renal Cell Carcinoma (RCC) drug had demonstrated enough benefits to outweigh the risk with clinical trial data included in its current NDA submission. The briefing documents, which go over the materials that were seen by the panel (and the public) two days ago and prior to the actual meeting, are here.
From Bloomberg:
“I cannot picture how I would talk with a patient about putting him or her on tivozanib, allowing them to live without progression longer but possibly to die faster,” Mikkael Sekeres, panel chairman and associate professor of medicine staff at the Cleveland Clinic’s Taussig Cancer Institute, said during the meeting today in Silver Spring, Maryland.
More specifically, there are major concerns about the uncertain improvements that the drug demonstrates in overall survival (OS) versus comparators, as shown in the clinical trial data from Phase III trial TIVO-1
From a AVEO and partner Astellas Pharma Inc. (TSE:4503) press release from February 12th, 2013:
The final OS analysis, as specified by the protocol, shows a median OS of 28.8 months (95% confidence interval [CI]: 22.5–NA) for tivozanib versus a median OS of 29.3 months (95% CI: 29.3–NA) for the comparator arm, sorafenib. No statistical difference between the two arms (HR=1.245, p=0.105) was observed.
This is graphically displayed on Page 67 of the panel’s briefing documents: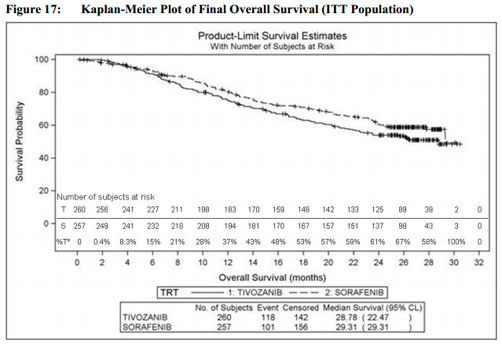
This vote virtually guarantees that the company will receive a CRL on the PDUFA date of July 28, 2013 although AVEO and its investors are hopeful that another Phase III trial can demonstrate improvement in a tivozanib arm versus a comparable VEGF inhibitor.
In response to today’s events, JPMorgan also downgraded AVEO from a “Overweight” to a “Neutral” rating as well.
Worth noting is the fact that AVEO had a stockholder’s equity figure of $118.9 M as of December 31st, 2012 as displayed in their 10-K filings. The market’s current valuation of the company implies that the tivozanib program is worth little to nothing at this point, which may not be the case if the drug can be salvaged later on. Having said this, the overwhelmingly negative vote will keep sentiment on the stock and company extremely negative until new clinical trial data arrives.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
AVEO Shareholders Get Scorched On Adcom Vote
Published 05/03/2013, 01:55 AM
Updated 07/09/2023, 06:31 AM
AVEO Shareholders Get Scorched On Adcom Vote
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.
