By Sarah Roden
On May 11, Intercept Pharmaceuticals Inc (NASDAQ:ICPT) reported first quarter earnings for 2015. The biopharmaceutical company does not yet have any approved products in its portfolio but is working on gaining approval for its lead candidate, OCA.
Highlights from the company’s quarterly report included a net loss of $39.4 million, or a loss of $(1.78) per share. This is a wider loss than the analyst consensus of $(1.69) but an improvement from $(12.61) in the same quarter of last year. CFO Barbara Duncan reiterated Intercept’s adjusted operating expense guidance for full-year 2015 of $180 million to $200 million.
Intercept Pharmaceuticals is developing obeticholic acid, or OCA, for patients with the liver disease PBC. OCA has received fast-track designation in the United States and orphan drug designation in both the U.S. and Europe. OCA for PBC is currently in the New Drug Application and the Market Authorization Application phase of filings, which is the final stage before commercialization. CEO Mark Pruzanski said in a conference call with investors that Intercept is on track to complete both applications this quarter. Intercept is also working on OCA in non-alcoholic steatohpatitis, or NASH, also known as fatty liver disease. OCA in NASH is in Phase 2 testing.
On May 11, Alan Carr of Needham reiterated a Buy rating on Intercept Pharmaceuticals with a $500 price target. Carr summarized that “OCA pre-launch activities in PBC are underway, including market research and market access.” Carr added that “MAA submission is also on track for this quarter” and expects it will launch commercially in the U.S. in early 2016 and in the E.U. later next year. Management plans to finalize “NASH Phase 3 trial design discussions with regulators in 2Q15” and expects top-line results “from the Phase 2 trial of OCA in NASH in Japan” by the end of 2015.
Alan Carr has rated Intercept Pharma 7 times since August 2013. He has a 71% success rate recommending the stock with a +79.8% average return per ICPT rating. Overall, Carr has a 73% success rate recommending stocks with a +38.7% average return per recommendation.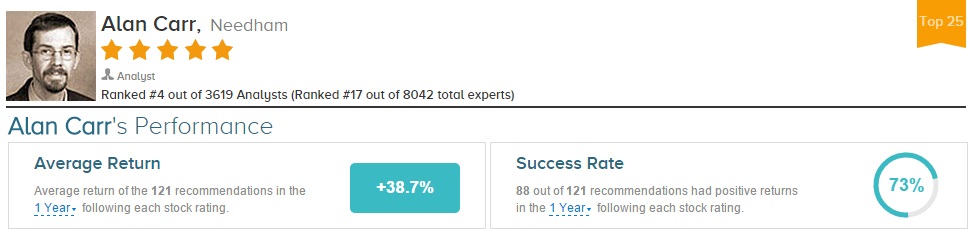
Separately on May 11, Liana Moussatos of Wedbush reiterated an Outperform rating on Intercept Pharmaceuticals with a $493 price target. Moussatos commented, “Phase 3 trial design for OCA in NASH is still on track for completion by the end of this quarter” and both “NDA and MAA submissions for OCA in PBC are expected to be completed this quarter.” The analyst expects OCA will launch in early 2016 and expects worldwide peak sales to reach $2.4 billion in 2022. Looking forward, Moussatos believes the announcement of the NDA for OCA will raise the price of the stock.
Liana Moussatos has rated Intercept Pharmaceuticals 16 times since April 2013, earning a 67% success rate recommending the stock with a +93.4% average return per ICPT rating. Overall, Moussatos has a 56% success rate recommending stocks with a +38.8% average return per rating.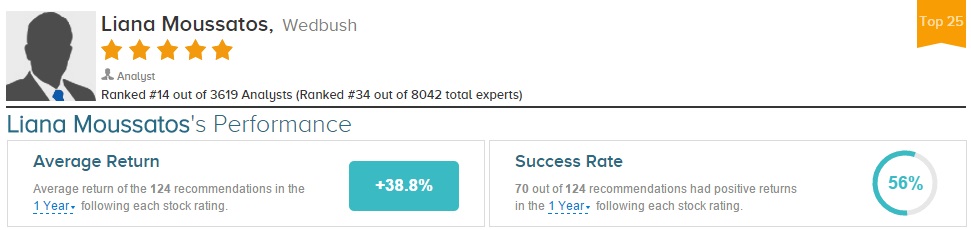
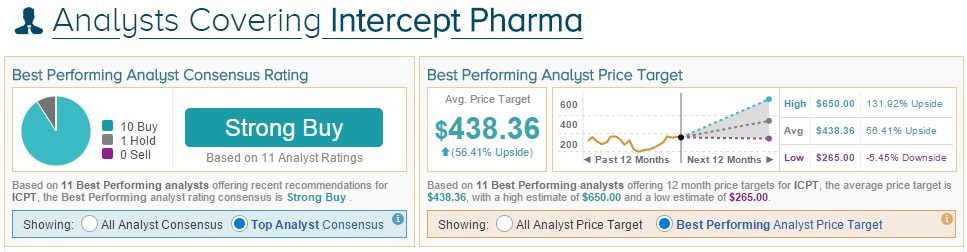
On average, the top analyst consensus for Intercept Pharmaceuticals on TipRanks is Strong Buy.