With all the recent drama surrounding Dendreon (DNDN) and their groundbreaking cancer drug Provenge, I believe it’s time to uncover a very Dendreon like biotech by the name of Agenus (AGEN). Agenus has been public for the past 11 years and has produced a slightly disappointing return of -99.4% in that time period. Despite its persistent under preformance Agenus offers an unprecedented amount of potential for those willing to take on a sizeable amount of risk. Generally speaking, small biotech companies are a joint effort of both a revolutionary (slightly mad) scientist and an investment banker, who together work to push a drug on the market. This would be called a One Trick Pony. Agenus is a Three Trick Pony.
Trick 1: Oncophage
This is Agenus’ blockbuster drug. It’s a cancer vaccine that is customized for each patient. The way it works is patients who have cancer (could be any kind, but at this moment tests are being done for glioma) get their tumor removed, then send it overnight to Agenus’ laboratory, out of the removed tumor Agenus creates a vaccine that gets shipped overnight back to the patient and then injected. At the moment there is nothing close Oncophage on the market other than Provenge. The reason it is so special is not only because of its outstanding efficacy (will expand on this later) but because it has no side effects. Unlike chemotherapy which can completely derail a patients life and has numerous serious side effects Oncophage has none. That’s right, there is no negative impact on the patient when they take Oncophage.
At this moment Oncophage is in Phase III tests and although it has been previously rejected by the FDA, there is significant hope that could be overturned. At the moment 8 institutions are enrolling in studies for Oncophage, this is just after a presentation at the American Society of Clinical Oncology from University of California in San Francisco reported outstanding results from Oncophage. On the drug patients survived an average of 47 weeks, which is 83.3% higher than the 26 week average for patients with glioma. If results anything close to this come out of the next studies being done on Oncophage then it will be hard for the FDA to keep Oncophage out of the US. Dendreon’s Provenge which just got approved in April 2010, and works very similarly to Oncophage only increases life expectancy of patients by 20%. This means Oncophage has 4x greater of an impact, and not only that but Oncophage is not limited to just one type of cancer, (Provenge is only prostate cancer) in the long run Oncophage could be used to treat any kind of tumorous cancer.
In terms of approval, at the moment Oncophage is only approved in Russia, but does have fast track and orphan drug approval in the US. The only thing holding Oncophage back is FDA approval. Just recently, Global Hunter Securities released a report on Agenus with a timeline including an initiation of discussion for Oncophage FDA approval in Q4 2011.
Trick 2: QS-21
QS-21 is an adjuvant, which means it’s put in vaccines to make them stronger so less of the important substances can be used (therefore more vaccines can be produced from less supplies). Currently there are over 20 vaccines that use QS-21 as an adjuvant. Many of them are in late stage development and could start producing revenue as soon as 2012-2013.
One of the more notable vaccines with QS-21 is a malaria vaccine GlaxoSmithKline (GSK) which happens to have just concluded Phase III testing, and will be evaluated sometime in early 2012. Another notable vaccine with QS-21 is a Johnson & Johnson (JNJ) Alzheimer’s vaccine currently in Phase II. There are also 3 other vaccines in Phase III testing with GlaxoSmithKline.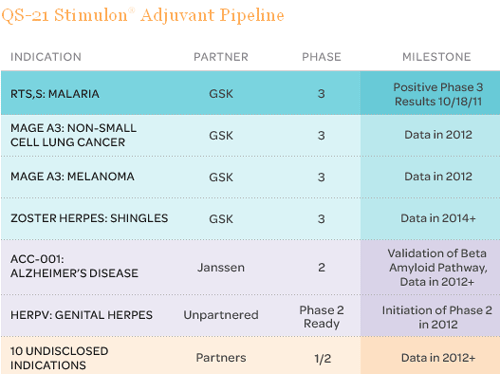
Trick 3: Herp-V
This drug is in the very early stages but at this point after outstanding Phase I results neglecting its value to Agenus doesn’t seem viable. Because Phase I results were so promising Agenus has decided to head right into initiation of Phase II testing in 2012.
The promise in this drug is enourmous, 1 in 6 adults have Herpes so the target market is vast. In the near term, from an investor prospective this could be an excellent short term catalyst if Phase II results come out anywhere near the caliber that Phase I delivered.
Valuation
Agenus is currently valued at $42 million and trading at $2.20 per share. To quickly put things in perspective, Dendreon was worth $6 billion before Provenge had FDA approval, and remember Oncophage has much larger potential than Provenge does. One main concern with Agenus from an investor perspective is their tendency to dilute their common stock. But even when that is calculated into their valuation the potential for huge returns is still very significant.
Of we assume Agenus’ number outstanding shares increases at 30% per year (approximately their current rate of dilution) then that means by the start of 2015 Agenus will have approximately 42 million shares outstanding, but let’s play it safe and assume that that number will be at 50 million by 2015. From dilution they will be able to raise somewhere around $40-50 million depending on where shares trade. Coincidentally $40-$50 million is exactly how much funding they will need if they keep burning cash at their current rate (and assuming still $0 revenue).
Global Hunter Securities uses 2015 for its time frame of Oncophage’s US approval, so I will too. We will assume that up until that point, all of Agenus’ other projects will have produced 0 revenue (even though this is unlikely because of the success of QS-21 in so many vaccines). Global Hunter Securities is estimating that Oncophage will generate $86 million in revenue in its first year of approval and increase to $215 million by its second year. If we use a conservative profit margin (of 45%) then that means in its first year of production Oncophage alone will produce $38.7 million in profit, or an EPS of $0.77 per share even with a 50 million share OS. Then in year two of Oncophage approval that profit will jump to $1.94 per share.
With shares trading at just $2.20 now, Agenus potential is off the charts. Dendreon is valued at 6-7x forward revenue (even though it has been valued as high as 15x that number). If Agenus were valued at just 5x 2016 revenue that would put a $1.1 billion dollar market cap on them in 2015. Which equates to $22 per share after accounting for dilution. But $22 seems very low considering Agenus will be profitable, and Dendreon on isn’t. So valuing on a PEG ratio or forward P/E would seem to be much more fitting. Considering profit growth will be around 30-60% for the 5 years after Oncophage hits the market ($1.5 billion in potential annual revenue for glioma alone), Agenus could be valued much closer to $90 per share (assuming 45% growth, 20 P/E, PEG ratio of 1 = $87.4, with 2016 EPS of $1.94).
Summary
Even by only conservatively calculating the value of Oncophage, Agenus could be worth $90 per share by 2015, if not sooner. If any of Agenus’ QS-21 projects, let alone Herp-V, follow through then things will look even brighter for this mini biotech wonder. It may take a while, but if things go their way, those holding Agenus stock will be rewarded greatly for their patience.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Agenus: The Three Trick Pony
Published 12/16/2011, 03:58 AM
Updated 07/09/2023, 06:31 AM
Agenus: The Three Trick Pony
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.
