Adventrx (ANX) has initiated patient recruitment in its pivotal Phase III study (EPIC) of ANX-188 in sickle cell disease, a significant achievement that puts the drug well ahead of its competitors. The trial design had previously been disclosed, which from an operational and statistical perspective appears to give the product an excellent chance of demonstrating a reduction in the duration of painful ‘crisis’ episodes. The rate of trial enrolment and patient dosing could have a bearing on Adventrx’s ability to secure a strategic partner and/or fresh finance required to complete the two-year study. We maintain our recently upgraded valuation of $137m or $2.50 per share.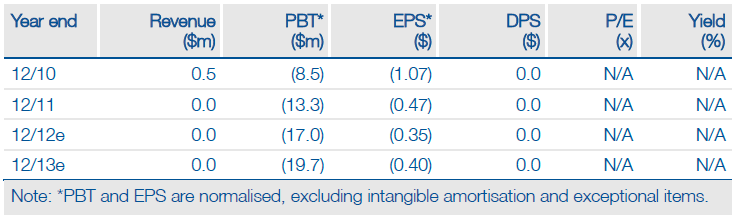
Well thought out
We recently reviewed (Neat design for ANX-188) the design of the EPIC (Evaluation of Purified 188 In Crisis) study for ANX-188 (purified poloxamer 188) that plays to the product’s strengths and addresses the inherent challenges in conducting clinical trials in sickle cell patients. The key takeaways are: a distinct patient population (children aged 8-17 years), a well-defined primary endpoint (duration of crisis is defined as the time from randomisation to the time of the last dose of parenteral opioid analgesic prior to hospital discharge), the selection of a clinically meaningful treatment benefit (16-hour reduction in crisis duration between ANX-188 and placebo), and sufficient power (388 patients with 90% power to detect a difference).
Increasingly attractive asset
ANX-188 is the only new molecular entity in Phase III for vaso-occlusive crisis in sickle cell disease and has the potential to become the first FDA-approved product in this setting for 15 years. The drug has orphan drug status (seven years market exclusivity in the US) and fast track designation (accelerated regulatory processes). It may also be entitled to earn a priority review voucher (applicable to rare paediatric diseases) which could then be sold on for a potentially significant amount of money. With estimated end-2012 cash of $35m unlikely to be sufficient to complete the EPIC study to results in H115 (clinical studies are also planned for ANX-188 in an additional indication), we view the start of EPIC and trial progress (recruitment/dosing) as determining factors in Adventrx securing a strategic partner(s) and/or fresh finance.
Valuation: Maintained at $137m or $2.50 per share
We maintain our recently upgraded valuation of Adventrx at $137m or $2.50 per share, which includes a US peak sales estimate of $85m and a 70% probability of success. Adventrx’s estimated FY12 net cash of $35m is sufficient for 18 months, covering EPIC and planned additional clinical studies of ANX-188. Securing a strategic partner and/or fresh finance for ANX-188 is the next potential major catalyst.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Adventrx Pharmaceuticals Pivotal Phase III Initiated
Published 01/31/2013, 06:11 AM
Updated 07/09/2023, 06:31 AM
Adventrx Pharmaceuticals Pivotal Phase III Initiated
An EPIC moment
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.