The review led by new CEO, Mr Colangelo, concluded that the REVIVE chronic limb ischaemia Phase III study will take too long to recruit and is beyond the financial resources available. With no immediate partner, Aastrom (ASTM) stopped the study. Aastrom is now reducing its headcount and cash burn by about 50%. The focus is now on the Phase II ischaemic cardiomyopathy (IDC) orphan indication. The ixCELL-DCM study will cost about $7m. If recruitment completes by early 2014, the data will presented in Q215. Funding of $35m until the end of 2015 will be needed.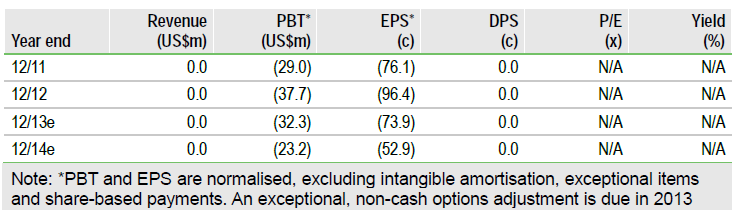
ixCELL-DCM: Ph II ischaemic dilated cardiomyopathy
IDC is a chronic indication where the heart distends and becomes weaker due to poor blood supply. The treatments are either transplant (rare) or, increasingly common, implanting artificial heart devices (LVAD). In the exploratory IMPACT study, ixmyelocel-T improved some indicators of heart performance. The Phase IIb, ixCELL-DCM study in 108 patients across 30 sites using catheter injection of cells. It is expected to enrol its first patient in early Q213 and complete recruitment in Q114, to present data in Q215. A Phase III will be needed; as IDC is an orphan condition, a single pivotal study might be run. Other orphan conditions are being evaluated and new indications for ixmyelocel-T are expected to be announced.
REVIVE is dead
The REVIVE study in chronic limb ischaemia was behind on patient recruitment, with only 40 patients in its first year out of 594. Given the cost and the uncertain time to completion, a partner was urgently needed to both fund the trial and to speed recruitment. As no immediate partner was found, REVIVE was stopped.
Financial implications and cash
In 2014, the cash use is expected to be about $15m per year. 2013 will be transitional with $6m in Q1 likely, $5m in Q2 including lay off costs and final REVIVE payments then about $8m for H2. Cash at the end of 2012 was $13.7m with $2.4m from an “At the Market” facility in Q1. Eastern Capital has first claim over about $44m of the value and holds over 22% of the voting rights. The Eastern pref stock converts at $3.50 per share. Funding of $35m will be needed until the end of 2015 to complete ixCELL-DCM and perhaps partner it for Phase III.
Valuation: A fundable high-risk trial
Edison has suspended its valuation of Aastrom until the situation is clearer. The ischaemic DCM indication is focused, potentially high-value but high risk.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Aastrom Biosciences: Financial Implications And Cash
Published 04/03/2013, 07:38 AM
Updated 07/09/2023, 06:31 AM
Aastrom Biosciences: Financial Implications And Cash
Focusing onto orphan diseases
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.