Yakult Honsha Co Ltd (T:2267), 4SC’s development partner for resminostat in Japan, has reached two clinical development milestones. First, just two weeks after it joined 4SC’s pivotal RESMAIN study (n=150) in CTCL, the company recruited the first patient in Japan. Top-line results from the RESMAIN trial are expected in mid-2019.
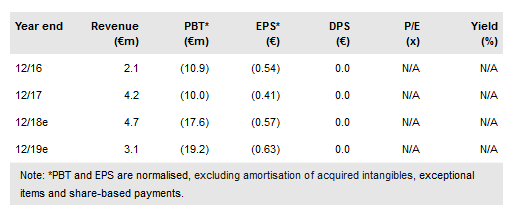
In addition, Yakult initiated its own Phase II study in biliary tract cancer (n=100) in combination with S-1 chemotherapy. S-1 is widely used in Japan and other Asian countries to treat patients following relapse after a 1st line chemotherapy regimen. The final data readout is expected in mid-2020. Meanwhile, at the AACR Annual Meeting in April, 4SC presented new preclinical data supporting the use of its second lead product, 4SC-202 in combination with various immunotherapy agents. Our valuation is virtually unchanged at €348 or €11.4/share.
New preclinical data with second lead asset 4SC-202
4SC has recently presented fresh preclinical data with 4SC-202 at the AACR Annual Meeting in Chicago, US on 14-18 April 2018. The findings show 4SC-202’s synergistic effect in combinations (double and triple) with various immunotherapy agents in animal cancer model. This backs the company’s previously announced intention to carry out a broad clinical programme for 4SC-202 involving combination studies with checkpoint inhibitors (CPI) to tackle the high non-responder issue.
Currently, 4SC-202 (HDAC class I specific inhibitor) is being studied in a Phase Ib/II trial SENSITIZE in unresectable melanoma in combination with pembrolizumab (Keytruda). The study is expected to be completed in H119 with top-line results from the first patient cohorts available in H218. Another investigator-led Phase II EMERGE study will test 4SC-202 in combination with the anti-PD-L1 antibody avelumab for treating GI tumours.
To read the entire report Please click on the pdf File Below: