Refocused and funded
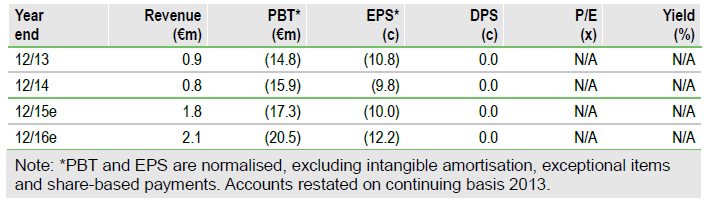
Tigenix (D) (BRU:G9U) FY14 results show a company now completely refocused on the proprietary allogeneic eASC technology platform and pipeline where the commercial potential is much greater. The ADMIRE Phase III results in fistulising Crohn’s disease in Q315 could lead to a possible EMA filing and a second, US Phase III; Lonza will produce the cells in the US. FY14 results show a reported loss of €11.4m, an operational cash outflow of €13.4m and year-end cash of €13.5m. A €25m non-dilutive funding was completed in Q115. The indicative value remains at €1.26 per share, but could rise to €1.82 per share if ADMIRE delivers significant Q3 data.
Focus on clinical-stage projects
The investment case for TiGenix has shifted to Cx601, with ADMIRE 24-week Phase III study data due in Q315 after 289 patients were recruited by 12 November. This trial is in perianal fistulising Crohn’s disease, comparing a 120m dose of expanded adipose stem cells (eASC) against placebo over 24 weeks. A Special Protocol Assessment (SPA) request on a Phase III design was submitted to the FDA in late 2014; feedback is expected in Q315. TiGenix aims to start a 180-patient Phase III in H216 once US contract manufacturing with Lonza is established and an IND is in place. Cx611, the intravenous eASC product, is in development for early rheumatoid arthritis and severe sepsis. Results from the Phase I sepsis challenge trial are expected in H115. A Phase IIa study in study in severe sepsis and a Phase IIb study in early RA are expected to start by the end of 2015.
Funding continuing operations
In 2014, to focus on the eASC projects, ChondroCelect was licensed to Sobi and the manufacturing facility sold to PharmaCell. The accounts have been rebased on a continuing operations basis. TiGenix received €338k in royalties in H214 covering the four months from September 2014; management notes that the implied sales of €1.54m were an increase over 2014. The company had €13.5m of cash on 31 December. A €25m, 9% convertible was issued in early March 2015. Management has stated that the company is now financed through to at least mid-2016.
Valuation: €1.26/share with cash to Q315
If Cx601 shows statistically significant efficacy, EU sales could start in 2017. We have maintained our indicative valuation of €1.26 per share based on sales forecasts to 2025. A 2025 multiple of 20x has been included to reflect continuing Cx601 sales and the potential of Cx611. The indicative value could rise to €1.82 per share on Cx601 success.
To Read the Entire Report Please Click on the pdf File Below