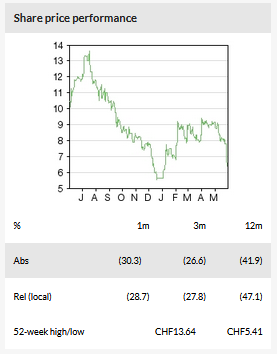
Newron Pharmaceuticals' (SIX:NWRN) plans for its novel mechanism of action drug, Evenamide, due to enter late-stage development for schizophrenia, have been delayed. Specifically, the FDA has requested that Newron carry out additional short-term safety studies before larger, pivotal-stage clinical trials can be undertaken. This has taken us and the market by surprise, as we had expected the two Phase II/III trials to start in Q219 as per guidance. The delay is a setback and we therefore push back our launch expectations by 18 months to 2024, erring on the side of caution. We now value Newron at CHF653m vs CHF714m previously.
Regression to Phase I studies unexpected
The emergence of safety concerns from preclinical models is unexpected given that positive clinical safety data were reported from the Phase I/IIa proof-of-concept study of Evenamide (March 2017). The concerns raised by the FDA related specifically to a chronic toxicity study in rats and CNS events observed in dogs at higher doses, which could be related to a class effect for the drug. Management will meet with the FDA to discuss what is required in more detail, but has indicated that additional preclinical and clinical safety studies (presumably Phase I in healthy volunteers) are likely to be required before a Phase III study is cleared by the regulators. We now estimate that registrational studies will not start until late-2020/early-2021, pushing back a potential launch to early-2024 (from late-2022).
Sarizotan and Xadago development plans on track
Newron remains well funded through key near-term inflections, including top-line data from the pivotal study of sarizotan in Rett syndrome (Q419) and its subsequent regulatory filing (2020), and the start of a label extension study for its commercial Parkinson’s disease (PD) drug Xadago for levodopa-induced dyskinesia (Q319). Newron reported net cash of €43.9m at end December 2018 and has access to an additional €40m loan facility through the European Investment Bank (EIB).
Valuation: CHF653m or CHF36.6/share
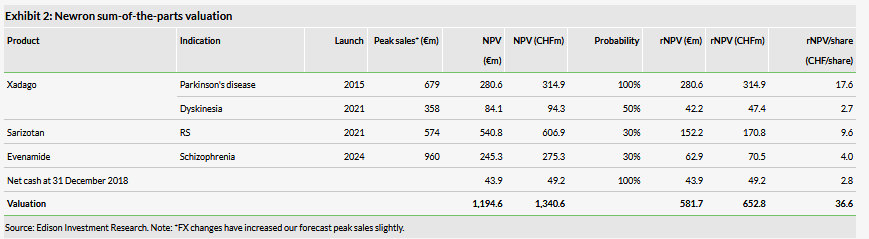
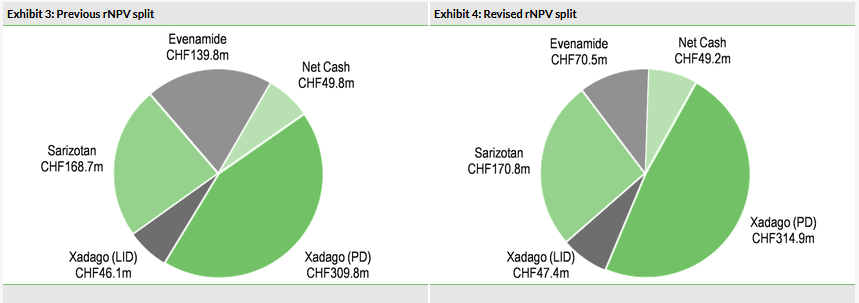
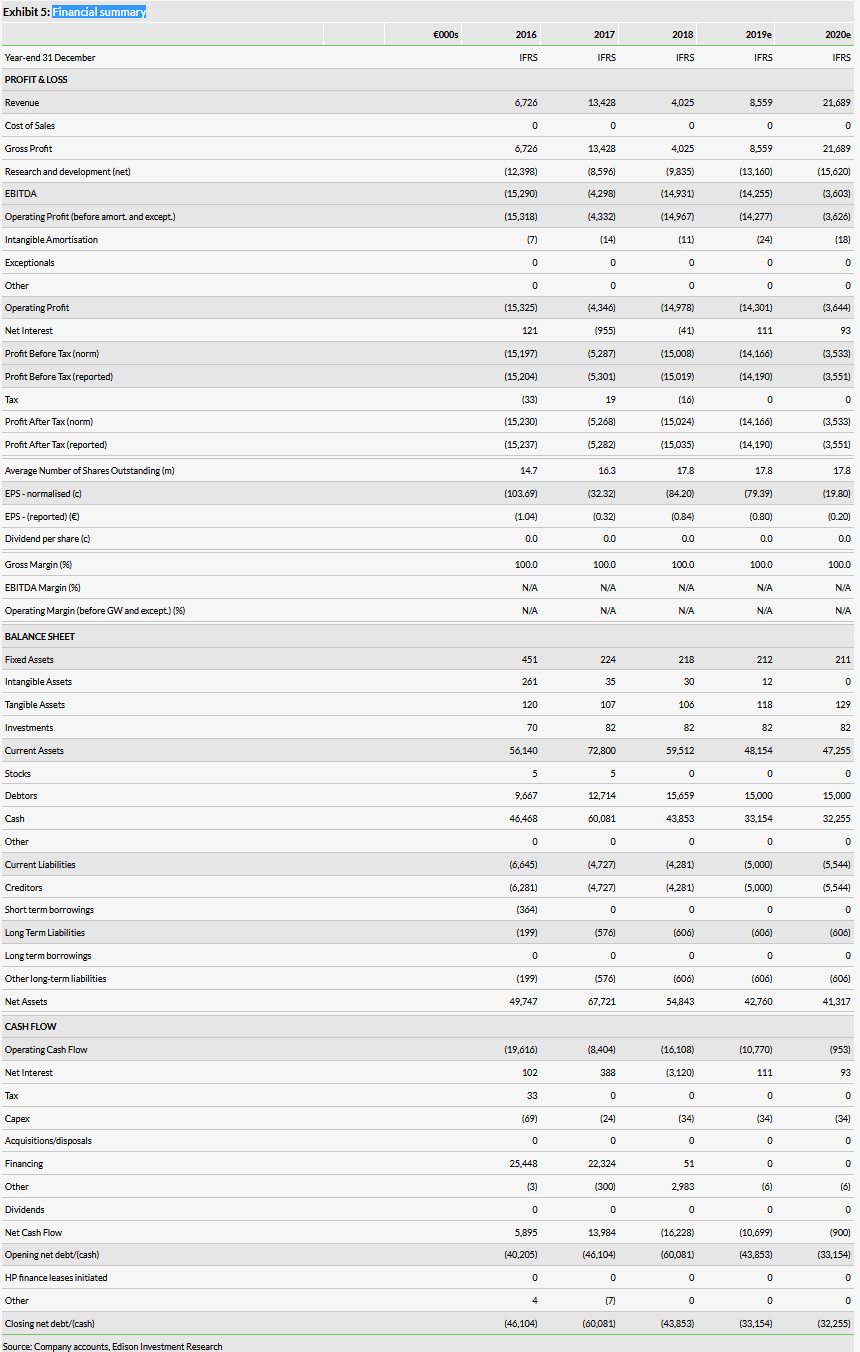
Our valuation of Newron has decreased to CHF653m from CHF714m. We have reduced the probability of success for Evenamide to 30% (from 50%), given that it is now likely to require an additional Phase I study, and pushed back its launch to 2024. Our valuation remains heavily skewed to Xadago and sarizotan, which represent 56% and 26% of our rNPV valuation, respectively. Sales of Xadago need to ramp up to reach our peak sales forecast of €679m.
Business description
Newron Pharmaceuticals is an Italian CNS-focused biotechnology company. Xadago (safinamide) for Parkinson’s disease has been launched in Europe and the US. Xadago is partnered with Zambon (EU), Meiji Seika (Japan), US WorldMeds (US), Seqirus (Australia/New Zealand) and Medison Pharma (Israel).
Safety first, safety second
Following the initial announcement, management followed up with an analyst call during which it highlighted that the concerns raised by the US FDA related to recently completed preclinical studies, including recent (undisclosed) findings from a long-term (26-week) toxicology study in rats and undisclosed CNS events observed in dogs on higher doses of Evenamide. These specifically related to Evenamide, as the studies did not relate to any combinations with atypical antipsychotics. Management has guided that these could be associated with commonly observed class effects related to the mechanism of action through which Evenamide works (inhibition of voltage-gated sodium channels). Management has guided that long-term preclinical toxic studies (26 to 52 weeks) are unlikely to be required and that sufficient preclinical safety data can be generated from short-term studies (four to eight weeks). However, the likely requirement to conduct another Phase I clinical study (50–60 healthy volunteers), even in parallel to preclinical work, will significantly affect development timelines; we note that the original Phase I study for Evenamide (conducted in 54 healthy volunteers) took 18 months to complete. With this in mind, we do not anticipate that Newron will be able start the originally proposed registrational studies for 18 months, which takes into account time frames to interact with the FDA, agree an appropriate study plan, analyse data and present the findings. Following receipt of further guidance from the FDA, we will revise these assumptions.
Cross-supportive studies remain the most likely path
Newron had been in active discussions with several regulatory bodies, including an end of Phase II meeting with the US FDA in Q218, to design two cross-supportive Phase IIb/III clinical trials for Evenamide in two schizophrenia patient populations, which Newron had previously expected to start in Q219 and complete in H220.
Study 003/005 for non-treatment resistant patients: chronic schizophrenic patients inadequately responding to atypical antipsychotic monotherapy (risperidone, aripiprazole, paliperidone, olanzapine or quetiapine). This trial will evaluate the efficacy, safety and tolerability of three fixed doses of Evenamide (or placebo) as an add-on to the patient’s current atypical antipsychotic medication.
Study 004/006 for treatment-resistant patients: defined as treatment-resistant schizophrenia (TRS) patients whose psychotic symptoms have failed two or more prior lines of pharmacotherapy and are not adequately responding to clozapine after 12 weeks. This trial will evaluate the efficacy, safety and tolerability of two fixed doses of Evenamide (or placebo) as add-on to clozapine.
We expect that Newron should be back on track to start these pivotal studies once the supportive explanatory studies have been completed. Regulators had indicated that positive results in both 003/005 and 004/006 would be enough to cover the efficacy requirements for submitting an NDA across both indications. If study 003/005 alone is positive, another confirmatory Phase III trial would be needed for this larger patient subset. We note that this scenario would require us to revisit our forecasts.

As an add-on to atypical antipsychotic drugs (APD) in the wider schizophrenia patient population, the scope for Evenamide is potentially huge and Newron would need to partner for the wider schizophrenia indication (add-on to any APD). However, Newron could elect to market the smaller indication of TRS as defined by resistance to clozapine by itself, and could rapidly capture this smaller subset of patients if breakthrough designation was granted.
Valuation
Our revised valuation of Newron is CHF653m (CHF36.6/share), down 8.5% from our previous valuation of CHF714m (CHF40.1/share) (Exhibits 3 and 4). This downgrade stems from reducing our probability of success for Evenamide to 30% from 50% to reflect the need for additional preclinical and Phase I studies, and delaying potential launch from late-2022 to early-2024. We have pushed back Evenamide-associated R&D to reflect the timing of the start of the larger, more costly Phase II/III programme. Additionally, we have rolled forward our model and updated FX rates. Our valuation includes Xadago peak sales in Parkinson’s disease, in addition to risk-adjusted contributions from Xadago for PD-related levodopa-induced dyskinesia (LID), sarizotan in Rett syndrome (RS) and Evenamide in schizophrenia. The breakdown of our rNPV valuation, using a 12.5% discount rate for clinical-stage assets and a 10% discount rate for commercially available asset Xadago, is shown in Exhibit 2.