AstraZeneca plc (NYSE:AZN) and partner Merck (NYSE:MRK) announced that the European Commission has approved their PARP inhibitor, Lynparza (olaparib) as a monotherapy, for a new patient population suffering germline BRCA-mutated HER2-negative locally-advanced/metastatic breast cancer, previously treated with chemotherapy. The drug is already marketed in the United States for the breast cancer indication.
Lynparza is also presently marketed for advanced ovarian cancer in the United States, the EU and across several other countries. With this latest nod in the EU, Lynparza can now be used to treat breast cancer patients, previously treated with an anthracycline and a taxane or who have progressed after being treated with endocrine therapy or are considered unsuitable for endocrine therapy.
This nod makes Lynparza the first approved PARP-inhibitor for treating metastatic breast cancer in Europe.
The approval was based on data from the phase III OlympiAD study, which evaluated Lynparza against chemotherapy. Results from the program showed that patients treated with Lynparza achieved an objective response rate (ORR) of 52% while the progression-free survival (PFS) was 7 months compared with 23% (ORR) and 4.2 months (PFS), attained by the chemotherapy arm, respectively. Moreover, Lynparza reduced the risk of disease progression or death by 42% as compared to chemotherapy.
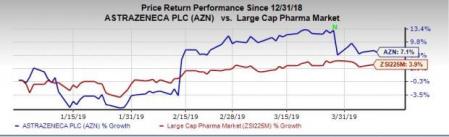
Shares of AstraZeneca have increased 7.1% in the year so far, outperforming the industry’s rise of 3.9%.
Other PARP inhibitors available in the market are Tesaro’s Zejula and Clovis Oncology, Inc.’s (NASDAQ:CLVS) Rubraca. Notably, Tesaro is now part of GlaxoSmithKline (NYSE:GSK) . However, both medicines are not approved for breast cancer.
Lynpazra is a key new product in AstraZeneca’s oncology portfolio alongside Tagrisso and Imfinzi. The drug delivered sales of $647 million in 2018, up 116% at constant exchange rate on its expanded use for ovarian cancer and the label expansion in breast cancer.
The approval of Lynparza last December as a first-line treatment of patients with BRCAm ovarian cancer in the United States and the drug’s latest label expansion in the EU is expected to support its further widespread application in 2019 and drive sales higher.
Zacks Rank
AstraZeneca currently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Zacks' Top 10 Stocks for 2019
In addition to the stocks discussed above, would you like to know about our 10 finest buy-and-holds for the year?
Who wouldn't? Our annual Top 10s have beaten the market with amazing regularity. In 2018, while the market dropped -5.2%, the portfolio scored well into double-digits overall with individual stocks rising as high as +61.5%. And from 2012-2017, while the market boomed +126.3, Zacks' Top 10s reached an even more sensational +181.9%.
Merck & Co., Inc. (MRK): Free Stock Analysis Report
AstraZeneca PLC (AZN): Free Stock Analysis Report
GlaxoSmithKline plc (GSK): Free Stock Analysis Report
Clovis Oncology, Inc. (CLVS): Free Stock Analysis Report
Original post
