Breathing space
Wilex AG O.N., (WL6G) is implementing significant cost reductions to extend its cash reach. Its focus has shifted onto its early-stage R&D pipeline, notably the potential of its innovative ADC technology and the preclinical service business. While partnering efforts for the late-stage pipeline have so far not been fruitful, Wilex now has greater flexibility to negotiate for the deals it seeks. Our valuation and financial forecasts are under review pending further guidance from the company.
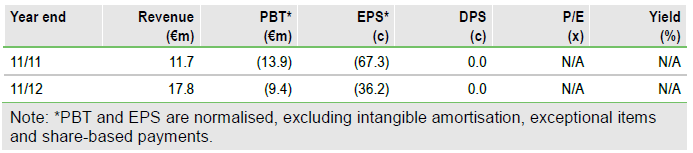
Cost reduction plan extends cash reach into H215
Wilex is scaling back its development activities and plans to reduce its Munich workforce from 51 to 10, extending its cash reach from H214 into H215. It will focus on early stage research activities at its Heidelberg Pharma subsidiary (42 staff), with its novel antibody drug conjugate (ADC) technology, and on developing its two small molecule oncology candidates from UCB.
Focus on ADC pipeline at Heidelberg Pharma
Heidelberg Pharma is developing its proprietary toxin-linker technology based on α-Amanitin, which has been shown to enhance the anti-tumour activity of antibodies. It has licensed use of the technology to Roche for upfront and milestone payments and aims to form new alliances. Separately, Wilex is developing WX-554 in Phase Ib/II and WX-037 in Phase I, both for solid tumours and expected to read out in H214. The products were in-licensed from UCB Pharma, a strategic investor in Wilex, which has buyback/co-development options. Wilex will discuss the next steps for these programmes with its partner in the next few weeks.
Upside from late-stage oncology pipeline if partnered
Wilex is in ongoing discussions to secure deals for its late-stage oncology programme, which have progressed more slowly than anticipated. However, Wilex is in a stronger position to negotiate with its longer cash runway. Phase III studies are required for Rencarex, for clear cell renal cancer (ccRCC) and for Redectane, a potential pre-surgical diagnostic for ccRCC. Mesupron was shown to enhance the activity of chemotherapy in Phase II trials in HER-2 negative breast cancer and pancreatic cancer, but requires confirmation in large-scale Phase III studies.
Valuation: Under review pending FY13 results
Our valuation is currently under review pending confirmation of the company’s financial guidance at FY13 results, notably the adjustment to its operating expense forecasts following the restructuring. Wilex will report its FY13 results before the end of March.
To Read the Entire Report Please Click on the pdf File Below