The US Phase II CALM study of intratumoural Cavatak in advanced melanoma has met its primary end point early, with a promising 33% response rate. The study remains on track for full enrolment in Q413 and should render final data by end-2014. Positive CALM data paves the way for a randomised Phase II study in late-stage melanoma and should increase partnering interest. Separately, the Phase I/II STORM study of intravenous Cavatak in advanced solid tumours is expected to start in Q413 following UK regulatory approval. Our valuation remains at A$61m.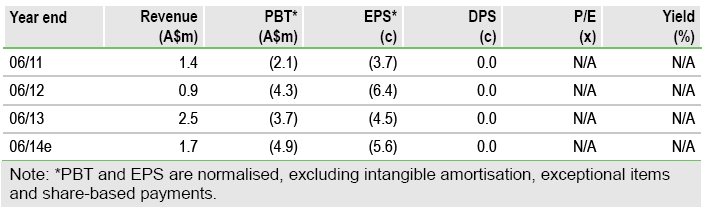
Phase II CALM study meets end point early
The US, single-arm Phase II CALM study of intratumoural (IT) Cavatak, as monotherapy in patients with late-stage (IIIc and IV) melanoma, has met its primary end point early with an encouraging immune related Progression Free Survival (irPFS) rate of 33% (10 out of first 30 evaluable patients). Moreover, the CALM data suggest that multiple dosing with IT Cavatak is well tolerated, with relatively mild adverse events (grade 1 or 2) and no serious AEs. CALM has now enrolled 44 patients and should reach its target of 54 evaluable patients by end 2013. Although final data are awaited (late 2014), the positive early readout supports progression into a randomised Phase II study and should increase partnering interest.
UK regulatory nod for Phase I/II STORM
Viralytics, (VRACY) has received approval from the UK Medicines and Healthcare products Regulatory Agency (MHRA) to initiate the two-stage Phase I/II STORM study of intravenous (IV) Cavatak in 30 patients with advanced solid tumours (melanoma, prostate, lung, bladder). Starting in Q413, the Phase I stage will administer Cavatak as monotherapy, while the second Phase II part will combine Cavatak with standard chemotherapy (docetaxel or carboplatin/paclitaxel) in the most responsive cancer type identified in Phase I. We expect the study to render initial data in 2014, which, if positive, could see Cavatak being developed further in other solid cancers.
Valuation: A$0.69/share (undiluted)
Our base case valuation of Viralytics remains unchanged at A$61m, or A$0.69/share (undiluted), derived using a risk-adjusted net present value method in only the lead indication of metastatic melanoma, and end-June 2014 net cash of c A$200k. We currently assume a 30% (Phase II) probability of success for IT Cavatak in melanoma. There is potential upside to our base case valuation if IV Cavatak delivers positive STORM data and, thus, is developed for a range of cancers other than melanoma.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Viralytics Clinical Trial Update
Published 09/24/2013, 08:09 AM
Updated 07/09/2023, 06:31 AM
Viralytics Clinical Trial Update
Cavatak Phase II meets primary end point
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.