Novartis’s decision could be for the best
Novartis is not in-licensing the rights to Transgene's (PARIS:TRNG) TG4010. Despite this setback, Transgene remains confident it will find a new partner, which is understandable given the new data from the PHASE II/III TIME trial. It is also possible that Transgene will find a better partner with a greater focus on oncology immunotherapies. It is advancing the TIME trial into the Phase III stage, and Pexa-Vec should enter Phase III in mid-2015 following the €65.5m capital raise in March and acquisition of its partner Jennerex by SillaJen. Our valuation of Transgene is €395m.
Novartis decides not to exercise licensing option
Novartis has decided not to exercise its exclusive option to in-license TG4010; no specific reason was disclosed. We believe that Novartis’s decision was due to strategic reasons as data from the TIME trials is impressive. Transgene reported in January 2014 that TG4010 reduced the risk of progression or death by >25% (HR<75%) in non-small cell lung (NSCLC) cancer patients with lower levels of the TrPAL biomarker in the first part of the Phase II/III TIME trial. Newly disclosed data show there was a HR of 0.71 (p=0.02) in all patients with non-squamous NSCLC, with an even better response in patients with normal TrPAL levels.
Transgene remains understandably confident
Transgene remains confident of finding a new partner by year end because of the data from the Phase II/III TIME study and plans to start the Phase III stage in H214. Transgene may also be able to find a better partner for TG4010. The prospects for TG4010 could be enhanced by licensing the product to a big pharma company with a core strength in immunotherapy (eg BMS or Merck).
Pexa-Vec set to enter Phase III
A Phase III in first-line hepatocellular cancer (HCC) with Pexa-Vec, an oncolytic virus, is due to start in mid-2015, with various other clinical trials being planned. Transgene and its partners are progressing into Phase III, despite the setback in September when the Phase II TRAVERSE trial in second-line HCC missed the primary endpoint, because of the strength of the data from eight other studies.
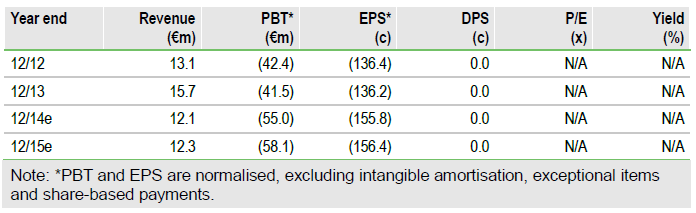
Valuation: DCF valuation of €395m, €10.83 per share
Our total valuation of Transgene is lowered by €67m to €395m. This takes into account Novartis’s decision, the €65.5m fund-raising (rights issue and private placement) and revisions to our launch date estimates for TG4010 and Pexa-Vec.
To Read the Entire Report Please Click on the pdf File Below