The recent failure of the Phase III PHOCUS trial is disappointing but we do not feel is critical to Transgene's (PA:TRNG) future. The company’s potential pipeline combined with ICIs remains the key driver of long-term value. Focus now turns to the rest of the pipeline, particularly the expected readout by year end of the Phase II trial of TG4010 + nivolumab in first-line non-small cell lung cancer (NSCLC). Early-stage development continues with the announcement of the Invir.IO collaboration with AstraZeneca (AZN) and the advancement of the first product candidate (TG4050) from the company’s myvac platform into the clinic (initiating clinical trials in H219). We value Transgene at €287m (€3.45/share) vs €312m (€5.02/share) previously.
Pexa-Vec fails Phase III futility analysis
The PHOCUS Phase III trial has been terminated based on the recommendation of the Independent Data Monitoring Committee (IMDC) following a planned interim futility analysis. The IMDC believed the trial was unlikely to meet its primary endpoint of a statistical increase in overall survival (OS) over the control arm. The trial had enrolled 190 patients at time of completion. Patients in the PHOCUS trial were randomised to one of two treatment arms where they received Pexa-Vec + sorafenib (Nexavar) or sorafenib alone. Following a review of the full data set and interactions with clinicians, Transgene has also decided to halt the ongoing Pexa-Vec + nivolumab Phase II trial.
Expansive pipeline offers opportunities
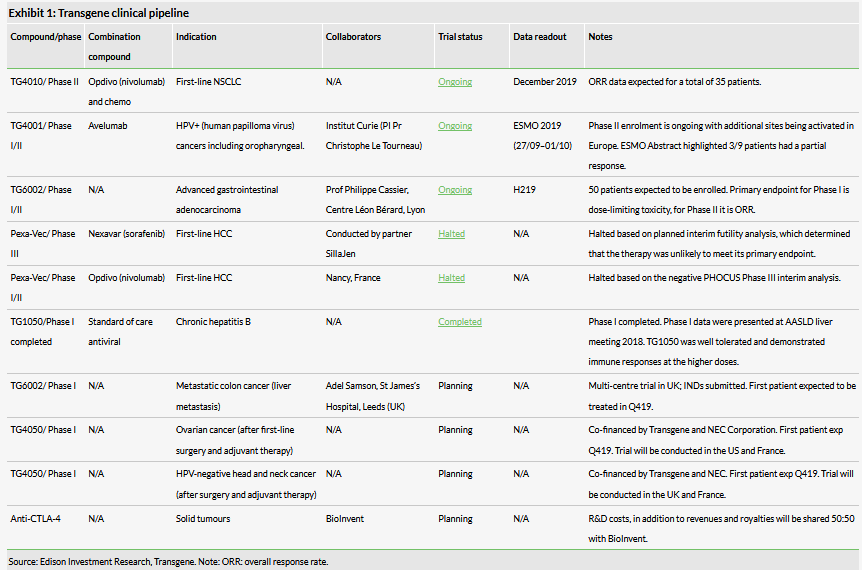
Transgene has four ongoing studies with two new products (TG4050 and anti-CTLA-4 oncolytic virus) expected to enter the clinic in the next 12 months. Efficacy data from the Phase II TG4010 (+nivolumab +chemotherapy) trial in first-line NSCLC will be central to determining Transgene’s long-term immune oncology (IO) strategy (data due in H219). Transgene continues to progress its technology; notably, in H119 it announced a partnership with AZN to develop five novel armed oncolytic viruses ($10m upfront, potential future milestones and royalties).
Valuation: €287m (€3.45/share)
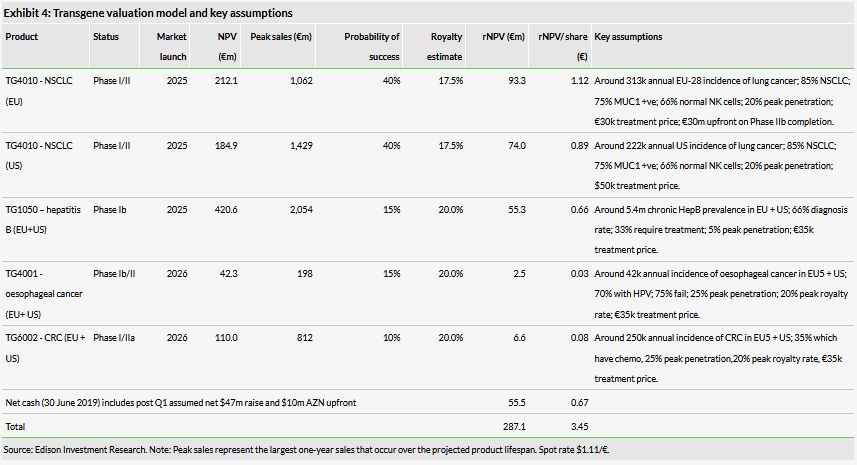
We value Transgene at €287m (€3.45/share) vs €312m (€5.02/share) previously, based on a risk-adjusted NPV model of TG4010, TG4001, TG1050 and TG6002. The decrease in value is driven by the removal of Pexa-Vec from our valuation. This has been offset by the gross €48.7m capital raise and $10m upfront from the AZN deal. We have updated our R&D cost assumptions across assets, rolled forward our model and updated for FX.
Share price performance
Business description
Transgene is a French drug discovery and development company focused on the treatment of cancer and infectious diseases with immunotherapies. Its products are TG4010, TG4001, TG1050, TG6002 and TG4050.
Investment summary
Company description: Immunotherapy platform
Transgene is a drug discovery and development company that develops viral vector-based immunotherapies for the treatment of cancers and infections. It has two platforms: therapeutic vaccines and oncolytic viruses. Its lead clinical-stage programme is TG4010. Therapeutic vaccine TG4010 is being trialled in combination with PD-1 ICIs in first-line non-squamous NSCLC. Transgene’s other clinical candidates are TG4001 in head and neck cancer, TG1050 in hepatitis B, TG6002 in glioblastoma and Pexa-Vec (+nivolumab) in hepatocellular carcinoma (HCC). Early-stage development continues with its Invir.IO (anti-CTLA-4) and myvac platforms (TG4050). Transgene is based near Strasbourg, France, and was founded in 1979. It was listed on the Nouveau Marché (now Euronext) in 1998. Transgene is 60% owned by Institut Mérieux.
Valuation: rNPV of €287m (€3.45/share)
We value Transgene at €287m (€3.45/share) vs €312m previously (€5.02/share), based on a risk-adjusted NPV model of TG4010, TG4001, TG1050, Pexa-Vec and TG6002. The decrease in value is driven by the removal of Pexa-Vec from our valuation. This decrease has been offset by the gross €48.7m capital raise (assumed net €47m) and $10m upfront from the AZN deal. We have updated our R&D cost assumptions across assets, rolled forward our model and updated for FX. Our valuation includes the prospects for TG4010 in NSCLC in the US and Europe (combined peak potential sales of c €2.5bn); TG1050 for hepatitis B in the US and Europe (peak potential sales of c €2.0bn across both regions); and TG4001 for oesophageal cancer in the US and Europe (peak potential sales of c €200m across both regions). Due to a shift in strategy we no longer value TG6002 for glioblastoma and instead now value its potential in colorectal cancer in the US and Europe (peak potential sales of c €800m across both regions). We do not currently include TG4050 or the Invir.IO anti-CTLA-4 oncolytic virus in our model but will reassess on the start of clinical trials.
Financials: €48.7m capital raise enables cash reach into 2021
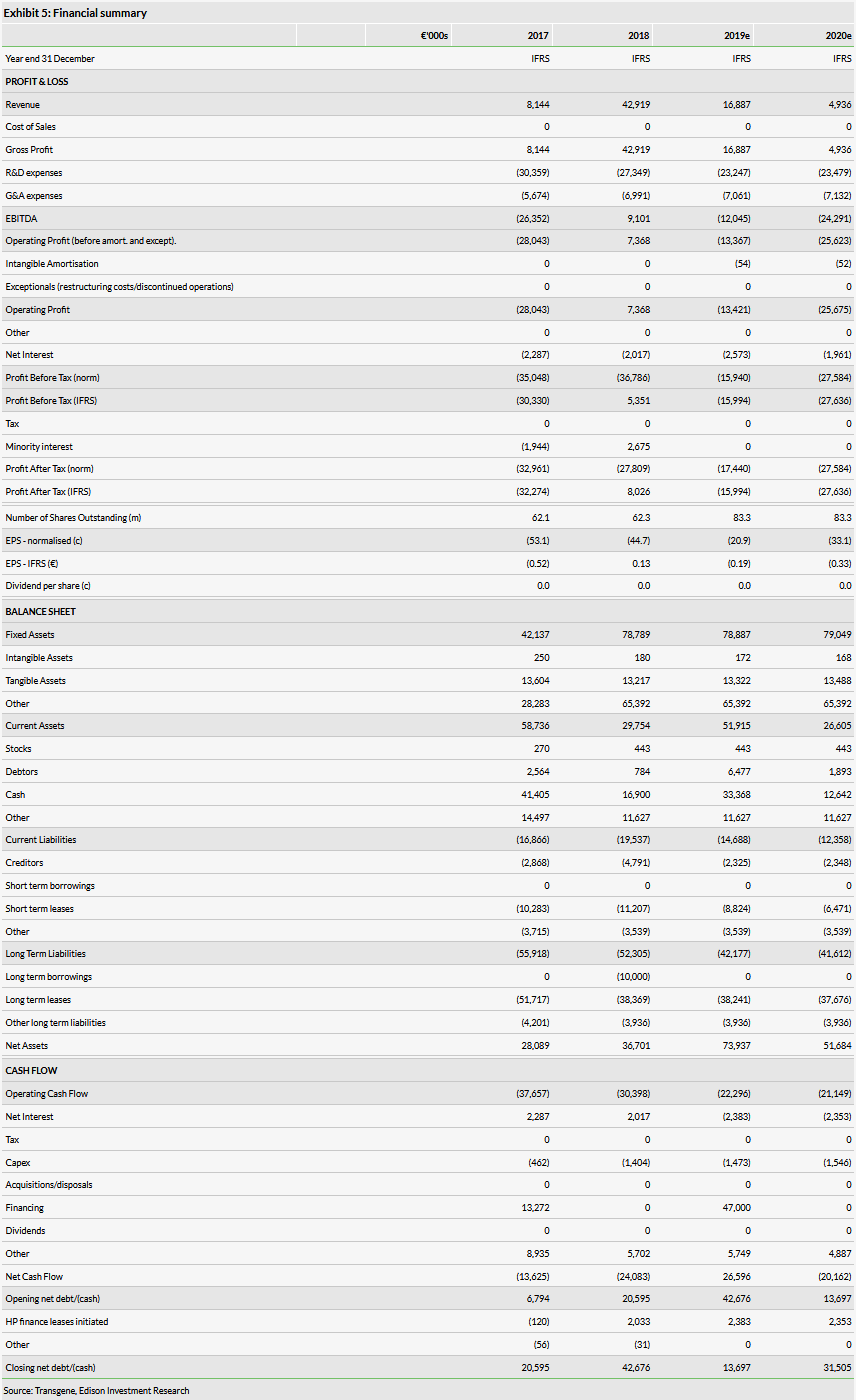
Transgene reported cash and equivalents of €12.8m at 30 June 2019 (vs €16.9m at 31 December 2018). This does not include the post period gross capital raise of €48.7m and the $10m upfront from signing the AZN deal. Net cash burn in H119 was €4.1m vs €8.3m in H119 and the company expects this to be around €20m in FY19 (we forecast €20.4m). Transgene has secured a €20m revolving credit facility with Natixis. The credit facility has a 30-month term and Transgene will be able to draw and repay the facility at its discretion. Transgene has used its shares in Tasly Biopharmaceuticals as collateral. We forecast a decrease in 2019 R&D based on a reduction in the number of clinical trials year on year while we expect 2019 G&A expenses to be largely in line with 2018. We forecast a cash reach into 2021 based on the capital raise and AZN upfront alone with no draw down of the Natixis credit facility.
Sensitivities: ICI combinations may not be conclusive
Transgene is subject to the usual risks associated with drug development, including clinical development delays or failures, regulatory risks, competitor successes, partnering setbacks and financing and commercial risks. The key sensitivities relate to the results of the ICI combination studies of TG4010 and TG4001. The outcome of the TG4010 combination studies in particular will have an impact on its partnership and/or the financing prospects for the programme. First results from these trials will be available later this year. We note that the ICI combination trials are small, open label and non-controlled; therefore, it is not possible to ascertain the magnitude of the effect of each product separately and assess the actual synergistic effect.
Broad pipeline of assets limits PHOCUS impact
Partner Sillajen has stopped the Phase III PHOCUS trial of Pexa-Vec in patients with advanced HCC (hepatocellular carcinoma) after planned interim futility analysis determined the therapy was unlikely to meet its primary endpoint. While the failure is disappointing, we do not feel it is critical to Transgene’s future. The potential of the company’s pipeline in combination with ICIs, particularly in relation to expanding the eligible patient population who respond to ICIs, remains the key driver of long-term value. As such, multiple combination readouts before year end will be more telling of the strategic potential of its assets, notably the data from the Phase II TG4010 (+nivolumab + chemotherapy) trial in first-line NSCLC.
In addition to clinical assets, Transgene continues to rapidly advance its next-generation platforms (Invir.IO and myvac). The first product candidate (TG4050) from the company’s myvac platform is expected to enter the clinic by year end, while the collaboration with BioInvent aims to have its anti-CTLA-4 oncolytic virus enter the clinic in Q120.
Global pharmaceutical companies continue to recognise the need to develop the next generation of immunotherapies. This was evidenced by the announcement earlier in the year of the collaboration with AZN to develop five novel armed oncolytic immunotherapies ($10m upfront, potential future milestones and royalties). This partnership provides external validation of Transgene’s oncolytic virus expertise and in the long term could be a significant contributor of revenue to the company.
Pexa-Vec: End of the line
Pexa-Vec is an oncolytic virus that targets fast-dividing cells with an active EGFR/Ras signalling pathway that is believed to cause cells to lyse and stimulate a T-cell immune response. Transgene’s partner Sillajen has stopped the Phase III PHOCUS trial of Pexa-Vec in patients with advanced HCC after a planned interim futility analysis determined the therapy was unlikely to meet its primary endpoint.
The PHOCUS study was an international randomised (1:1) open-label study comparing Pexa-Vec followed by Bayer’s sorafenib (anti-BRAF/VEGFR/PDGFR tyrosine kinase inhibitor) versus sorafenib alone in patients with advanced HCC who have not received prior systemic therapy (n=600). Pexa-Vec was administered as three bi-weekly intratumoural injections at day one and weeks two and four, followed by sorafenib at week six; the comparator arm received sorafenib 400mg twice daily starting on day one. The primary endpoint was OS; secondary endpoints include time to progression, progression-free survival (PFS), overall response rate (ORR) and disease control rate (DCR). SillaJen had responsibility for conducting and funding the study and retains rights outside Europe, China and South Korea. Transgene retains development and commercialisation rights in Europe, Lee Pharma retains China rights and Green Cross Pharma has South Korea rights.
The trial was ceased based on the recommendation of the IMDC. The IMDC believed the trial was unlikely to meet its primary endpoint of a statistical increase in OS over the control arm. The trial had enrolled 190 patients at the time of completion. Patients in the PHOCUS trial were randomised to one of two treatment arms where they received Pexa-Vec + sorafenib (Nexavar) or sorafenib alone.
Following the failure of the Phase III PHOCUS trial, analysis of the data and conversations with physicians in the field, Transgene has decided to cease the Pexa-Vec + nivolumab (Opdivo) Phase II trial. We believe recent struggles for ICIs in HCC, as shown by the failure of the second-line Phase III KEYNOTE-240 trial (following accelerated approval of Keytruda in this setting based on Phase II KEYNOTE-224 results) and the first-line Phase III Checkmate-459 trial, will also have been factored into the reasoning for the cessation of the trial, particularly as the standard of care (SOC) for HCC continues to evolve rapidly, with recent approvals for tyrosine kinase inhibitor lenvatinib (first-line HCC) and cabozantinib (second-line HCC).
At this time we do not expect Pexa-Vec to be utilised in any new clinical trials.
TG4010: Important data readout approaches
Initial data from the Phase II (NCT03353675) in first-line patients testing a triple combination of TG4010, Opdivo (nivolumab, Bristol Myers Squib) and chemotherapy are expected to readout by year end. The trial is a single-arm EU/US study and is anticipated to enrol 39 patients (without EGFR mutations or ALK rearrangements) who express low or undetectable levels of PD-L1. The primary endpoint is an objective response rate and Transgene is funding the study with Bristol Myers Squib supplying Opdivo. For patients without a driver mutation (EGFR, MEK etc) the standard of care has quickly become chemotherapy plus Keytruda, irrespective of PD-L1 status. The first-line setting could be a significant opportunity for Transgene if TG4010 in combination with a PD-(L)1 ICI is proven to be more effective than PD-(L)1 ICI monotherapy treatment in low PD-L1 expressing patients.
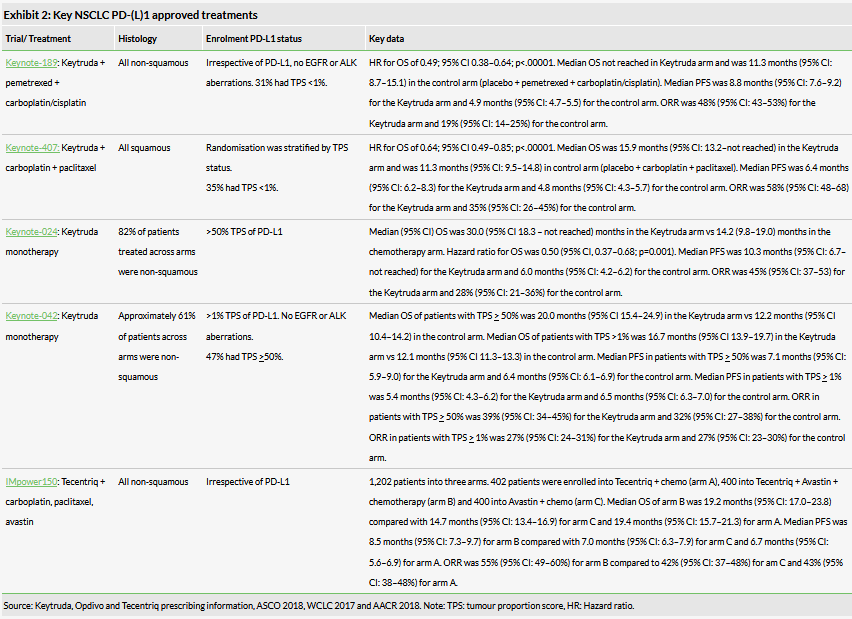
First-line NSCLC treatment is now dominated by PD-(L)1 approvals, notably Keytruda plus chemotherapy and Tecentriq plus Avastin plus chemotherapy. Transgene’s first-line TG4010 NSCLC trial will need to demonstrate a comparable or improved ORR (Keytruda combo ORR: 48%, 95% CI: 43–45% and Tecentriq triple combo ORR: 55%, 95% CI: 49–60%) in similar patient populations to be considered for future development. Since the commercial launch of Keytruda (Merck) and Opdivo (Bristol Myers Squib) in melanoma in 2014, PD-(L)1 ICIs have become the standard of care in many cancers (FY18 sales of Opdivo and Keytruda were $6.7bn and $7.2bn respectively).
Transgene believes its therapeutic vaccine TG4010, a modified vaccinia Ankara (MVA) virus that expresses MUC1 antigen and interleukin-2 (IL-2), could prove beneficial in combination with PD-(L)1 ICIs. TG4010 is believed to induce a specific T-cell response to target MUC1-expressing cancer cells that when used in combination with a PD-(L)1 ICI removes inhibitory effects on the T-cell. Along with the MUC1 T-cell stimulation, IL-2 enables the differentiation of T-cells into effector and memory T-cells, which could enhance the response against the MUC1-expressing cancer cells.
TG4010 has previously been tested in combination with chemotherapy. Data from the Phase IIb TIME trial compared chemotherapy plus TG4010 to chemotherapy plus placebo in patients with advanced NSCLC (n=222). For the total population (squamous and non-squamous NSCLC), patients receiving TG4010 benefited compared to the placebo arm (ORR: 39.6% vs 28.8%; duration of response: 30.1 vs 18.7 weeks). In non-squamous NSCLC patients, the ORR was 40% (n=98) in the experimental arm compared with 28% in the control arm, with median duration of response of 41 and 18 weeks respectively. Median PFS in non-squamous NSCLC patients was 5.8 months (95% CI: 5.5–7.2) vs 5.0 months (95% CI: 4.2–5.8) for the control. The median OS in non-squamous NSCLC patients was 14.6 months (95% CI: 11.1–20.4) vs 10.8 months (95% CI: 9.5–14.5) for the control. In a post-hoc analysis of all non-squamous NSCLC patients, there was a similar level of PFS and OS benefit in the 97 patients with low levels of PD-(L)1 expression (
Rest of pipeline still on track for inflection points
Transgene has a range of other product candidates in the clinic with multiple readouts anticipated in the next 12 months. Transgene forecasts readouts for TG6002 in colorectal cancer (administered intravenously) and TG4001 in HPV+ (human papilloma virus) cancers including oropharyngeal (in combination with PD-L1 inhibitor avelumab) in H219 and H1120 respectively. Additionally, Transgene will present Phase Ib trial data at ESMO 2019 (27 September to 1 October) for TG4001. Beyond these, new trials are expected to initiate by year end with TG4050 tested in patients with HPV+ head and neck/ovarian cancers and TG6002 tested in patients with colorectal cancer and liver metastasis (intrahepatic arterial administration).
TG4001: Potential in HPV-positive HNSCC
TG4001 is a therapeutic vaccine based on an MVA vector engineered to express HPV 16 antigens E6 and E7 with adjuvant IL-2. In collaboration with Pfizer (NYSE:PFE) and Merck, Transgene is running a Phase Ib/II study in second-line HPV-positive head and neck squamous cell carcinoma (HNSCC) combining TG4001 and the anti-PD-L1 antibody avelumab. The trial is funded by Transgene, while Pfizer and Merck will provide avelumab. Pfizer and Merck do not have exclusive rights to the data or TG4001, both of which remain Transgene’s. Patient enrolment is ongoing with 52 patients expected to be treated. The study is split into two parts: in the Phase Ib component nine patients were tested at increasing doses of TG4001 in combination with a fixed dose of avelumab.
Transgene will present Phase Ib trial data at ESMO 2019 and plans to host a press conference on 3 October to further detail the findings. The published ESMO abstract highlights the treatment of nine patients with oropharyngeal (five), anal (two), cervical (one) or vaginal (one) cancer. Patients had refractory or metastatic cancers and had received at least two prior treatment regimens. Three patients received a low dose (5x106 plaque forming units (pfu)) of TG4001 and the other five a high dose (5 x107 pfu). TG4001 was administered SC weekly for six weeks, every two weeks up to M6, and every 12 weeks thereafter. All patients additionally received avelumab at 10mg/kg every two weeks. No dose-limiting toxicities or serious adverse events were observed. Three out of the nine patients had a partial response. Phenotypic and gene findings supported the idea of a shift in tumours from a ‘cold’ state to an immunologically ‘hot’ state.
In the expansion cohort (Phase II), 40 patients with HPV-positive HNSCC are being enrolled into a single-arm cohort. Patients will receive the highest dose tested in the Phase Ib component of the trial. The primary outcome of the Phase II part will be efficacy as measured by ORR. Interim results are expected in H120. Details of the financial terms, IP rights or other aspects of the agreement between the three parties have not been disclosed.
TG1050: Phase I complete in hepatitis B
TG1050 is a therapeutic vaccine for the treatment of chronic hepatitis B that expresses three antigens of the hepatitis B virus (HBV). Transgene presented Phase I data (NCT02428400) for TG1050 at the AASLD 2018 liver meeting. Data were presented on the safety and immunogenicity of either single or multiple injections of TG1050 at various dose levels. The 48 patients were split into single (n=12) or multiple injections (n=36) cohorts. Patients in each cohort were then randomised across three dose levels (109, 1010 and 1011 virus particles) and randomised in each dose level 3:1 to either receive treatment or placebo.
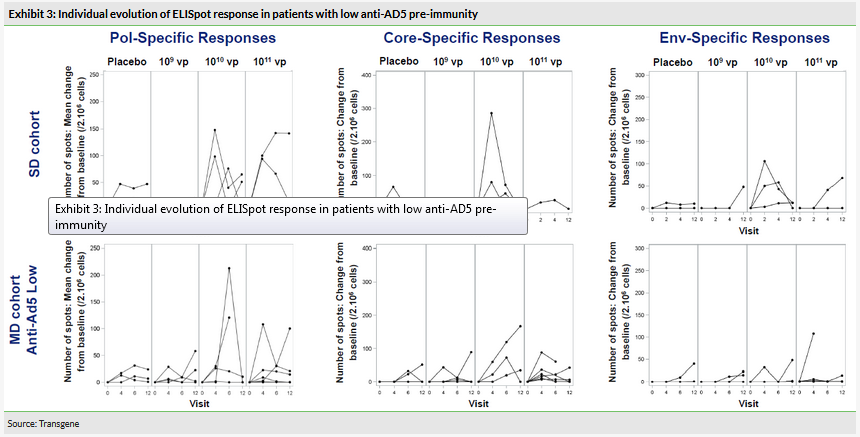
There were no serious adverse events (AE) reported and all AEs were grade 1 or 2 except one grade 3, which was not related to treatment. Immune responses to the HBV antigens that TG1050 encodes for were monitored (Exhibit 3). Detectable responses occurred at the higher doses (1010 and 1011 virus particles) in both the single injection (SD) cohort and the multiple injection (MD) cohort against the three antigens vectorised by TG1050. The intermediate dose (1010 vp) was immunogenic in c 70% of patients.
Additionally, at the 2018 AASLD liver meeting Transgene presented preclinical data on the combination of TG1050 with a range of immunomodulators (TLR9 agonist, PDE5 inhibitor) and direct acting antivirals (siRNA, capsid inhibitor, entecavir). Combinations with the TLR9 agonist, PDE5 inhibitor and the siRNA led to improvements in antiviral effects.
Next-generation viral vector in TG6002
TG6002 is a viral vector derived from vaccinia virus expressing the FCU1 gene. The FCU1 gene encodes a protein that catalyses the transformation of the nontoxic pro-drug flucytosine into 5-FU and 5-fluorouridine monophosphate, a widely used chemotherapy. Its expression is restricted to tumours, thereby reducing toxicity to normal tissues. The company has conducted numerous in vitro and in vivo experiments to establish its mechanism of action.
TG6002 is involved in a Transgene-sponsored Phase I/II trial in advanced gastrointestinal adenocarcinoma (colon cancer) and the first patient was dosed (via IV) in October 2018. The trial (NCT03724071) is enrolling 50 patients who have failed, or are intolerable to, standard therapeutic options. Initial data are expected by year end.
Additionally, Transgene is preparing a new Phase I/II study in patients with advanced gastrointestinal adenocarcinoma (colon cancer) with liver metastasis who will be dosed by intrahepatic arterial administration. The first patient is expected to be enrolled by year end. Transgene also expects to take TG6002 into a new indication in 2020.
We note the Phase I/II trial in glioblastoma with Assistance Publique Hôpitaux de Paris (principal investigator Professor Delattre) and support from French National Cancer Institute is still enrolling patients. However, it no longer forms a core part of Transgene’s strategy. No safety issues have been found to date; according to clinicaltrial.gov the completion of the trial is expected in 2021.
Evolving partnerships and platforms
Transgene continues to advance its partnerships and early-stage technologies. Notable in 2019 was the announcement of a partnership with AZN to develop oncolytic viruses using Transgene’s Invir.IO platform. Although early, the partnership could generate significant value for Transgene in the long run. More advanced is Transgene’s partnership with Tasly Biopharmaceuticals, which was formed in summer 2018. Tasly paid Transgene $48m in shares and has gained Greater China rights to T601 and T101. The Invir.IO collaboration with BioInvent has been expanded to include the development of anti-CTLA-4 antibody armed oncolytic vaccinia virus (expected in the clinic in H120). The first product candidate (TG4050) from the company’s myvac platform is expected to enter clinical trials later this year.
AZN partnership major validation of Invir.IO platform
Global pharmaceutical companies continue to recognise the need to develop the next generation of immunotherapies. This was evidenced by the announcement earlier in the year of the collaboration with AZN to develop five novel armed oncolytic immunotherapies based on Transgene’s novel vaccinia virus double-deleted backbone (Invir.IO platform). Transgene received $10m upfront and is eligible for additional pre-clinical milestones of up to $3m. If AZN decides to exercise an option on any of the candidates, Transgene will receive an option payment in addition to eligibility for development and commercial milestones, plus royalties on any future sales.
Transgene is responsible for the design and engineering of the oncolytic virus and for in vitro pre-clinical development. AZN will select the genes to be encoded within the viruses and will be responsible for further in-vivo pre-clinical development. If AZN decides to take up the option on a virus it will then be responsible for clinical development and commercialisation. This partnership provides external validation of Transgene’s oncolytic virus expertise and in the long term could be a significant contributor of revenue to the company. We do not currently value the AZN partnership due to its early nature but will reassess this once assets enter the clinic.
Tasly Biopharmaceuticals: Hong Kong IPO still expected
In July 2018, Transgene sold the greater China rights for T601 (TG6002) and T101 (TG1050) to Tasly Biopharmaceuticals. This was an expansion of a previous partnership formed in 2010 that was based on the development of T601 and T101. Transgene received approximately 2.53% of the outstanding capital of Tasly Biopharmaceuticals, which was valued at $48m. However, the valuation of this holding could decrease or increase depending on future funding events. Tasly has filed its prospectus with the Hong Kong Stock Exchange and we expect it to aim complete the IPO as soon as possible, although we note local political unrest could halt or delay any planned filing. Transgene has a 12-month post IPO lock up on its shares in Tasly Biopharmaceuticals.
In China T101 has completed a Phase I trial of T101 for treating chronic HBV infection. T101 is a viral vector that expresses the same suite of HBV antigens as TG1050. The trial is a randomised, double-blind, placebo-controlled study that enrolled 36 patients who are on antiviral therapy. According to Tasly the trial ‘produced satisfying results in safety, tolerability and immunogenicity’ with both single and multiple injections well tolerated and producing no serious AEs. The trial demonstrated that T101 could stimulate an HBV-specific T-cell response when injected subcutaneously generated antiviral activity. Future development plans are unclear at this time.
T601 is an oncolytic virus that expresses the same FCU1 gene as TG6002. Tasly submitted an IND application to the China authorities (National Medical Products Administration) in April 2018 and it was approved in April 2019. Under the IND application, Tasly has agreed to commence a clinical trial in advanced malignant solid tumours of the digestive tract. The company expects a Phase I trial to initiate in H219 and plans to enrol 45–60 patients.
Invir.IO and myvac: Deals demonstrate value
Transgene’s early-stage technology platforms Invir.IO and myvac are coming to a critical juncture in the next 12 months as they enter the clinic. For myvac, Transgene has announced its plans to start clinical development (partnership with NEC Corporation) for new asset TG4050 by year end. TG4050 is based on Transgene’s personalised immunotherapy platform (myvac) and NEC’s neoantigen prediction system. Transgene will initiate two clinical studies in H219 focusing on second-line (following surgery and chemo) ovarian cancer and second-line (following surgery and radiation therapy) head and neck cancer patients. The trials are jointly funded by both parties.
In additional support of the industrialisation of the myvac platform, the NEOVIVA project was awarded a €5.2m grant from Bpifrance’s ‘Investments for the Future’ programme. Transgene will receive €2.6m of the grant. The NEOVIVA project is across four companies and aims to develop and validate manufacturing approaches for individualised therapies. The two TG4050 trials will provide proof of concept for the myvac platform.
In 2017, Transgene launched its Invir.IO technology platform. The technology aims to use the cancer cell-killing capability of oncolytic viruses in combination with the ability of the viruses to insert relevant genes into cancer cells to express other anti-cancer compounds. The technology is based around high-capacity vaccinia viruses, which are engineered to express a range of anti-cancer drugs such as ICIs, enzymes, ligands, chemokines and cytokines. Transgene is developing both its own internal proprietary product candidates and partnered candidates, most notably with AZN and BioInvent.
The BioInvent collaboration is focused on new, unspecified multifunctional oncolytic viruses for the treatment of solid tumours. Transgene will provide its oncolytic virus expertise and technology (Invir.IO), while BioInvent will provide its antibody capabilities, including novel antibody sequences. Costs, revenue and royalties will be split 50:50. The first product will look to vectorise BioInvent’s anti-CTLA-4 antibodies for use in cancers. R&D costs, in addition to revenues and royalties from any candidates produced, will be shared 50:50. Transgene has 10 wholly owned preclinical candidates based on its Invir.IO platform and anticipates the first product candidate will enter the clinic in H120.
Sensitivities
Transgene is subject to the usual risks associated with drug development, including clinical development delays or failures, regulatory risks, competitor successes, partnering setbacks and financing and commercial risks. The key sensitivities relate to the results of the ICI combination studies of TG4010 and TG4001. The outcome of the TG4010 combination studies in particular will have an impact on its partnership and/or the financing prospects for the programme. First results from these trials will be available later this year. We note that the ICI combination trials are small, open label and non-controlled; therefore, it is not possible to ascertain the magnitude of the effect of each product separately and assess the actual synergistic effect.
Financials: €48.7m capital raise boosts cash reach
Transgene reported revenue for H119 of €4.9m (vs €3.5m in H118), which consisted of €3.1m in government financing for research expenditure and €1.5m in collaboration and licensing income. Net cash burn in H119 was €4.1m vs €8.3m in H119 and the company expects this to be around €20m for the full year (we forecast €20.4m).
Transgene reported cash and equivalents of €12.8m at 30 June 2019 (vs €16.9m at 31 December 2018). This does not include the post period gross capital raise of €48.7m and the $10m upfront from signing the AZN deal.
Transgene has secured a €20m revolving credit facility with Natixis. The credit facility has a 30-month term and Transgene will be able to draw and repay the facility at its discretion. Transgene has used its shares (27.4m) in Tasly Biopharmaceuticals as collateral. We forecast a decrease in 2019 R&D (to €23.3m) based on a reduction in the number of clinical trials y-o-y as numerous trials were completed or terminated in 2018 (TG4010 in second-line NSCLC, TG1050 in hepatitis B and Pexa-Vec in HCC, breast and solid tumours). We forecast 2019 G&A expenses (€7.1m) to be largely in line with 2018. We forecast a cash reach into 2021 based on the capital raise and AZN upfront alone with no draw down of the Natixis credit facility.
Valuation: €287m (€3.45/share)
We value Transgene at €287m (€3.45/share) vs €312m (€5.02/share) previously, based on a risk-adjusted NPV model of TG4010, TG4001, TG1050 and TG6002. The decrease in value is driven by the removal of Pexa-Vec from our valuation. This has been offset by the gross €48.7m capital raise (assumed net €47m) and $10m upfront from the AZN deal. However, we note that on a per-share basis our valuation has lowered significantly as a result of the dilution from the €48.7m capital raise. We have updated our R&D cost assumptions across assets, rolled forward our model and updated for FX. Our valuation includes the prospects for TG4010 in NSCLC in the US and Europe (combined peak potential sales of c €2.5bn); TG1050 for hepatitis B in the US and Europe (peak potential sales of c €2.0bn across both regions); and TG4001 for oesophageal cancer in the US and Europe (peak potential sales of c €200m across both regions). Due to a shift in strategy we no longer value TG6002 for glioblastoma and instead now value its potential in colorectal cancer in the US and Europe (peak potential sales of c €800m across both regions). We do not currently include TG4050 or the Invir.IO anti-CTLA-4 oncolytic virus in our model but will reassess on the start of clinical trials
Our key assumptions on TG4010, TG1050, TG6002 and TG4001 are as follows:
TG4010: we model a classical clinical development timeline for the project in NSCLC. There could be considerable upside if the company goes for accelerated filing, and/or development is expanded into other cancer indications.
TG1050: our valuation includes the EU and US markets and we have assumed TG1050 will be out-licensed on completion of a successful Phase II proof-of-concept study. We forecast that a partner will fund Phase III trials, registration and commercial launch.
TG6002: we no longer value glioblastoma and our valuation is now based on colorectal cancer patients in the EU and US. For TG6002 we have assumed that TG1050 will be out-licensed on completion of a successful Phase I studies. We forecast that a partner will fund Phase II/III trials, registration and commercial launch.
TG4001: our valuation is based on HPV-positive oesophageal patients in the EU and US who have failed standard therapy.