Interim Phase II data for Transgene's (TNG.PA) recent clinical trial provided further evidence of JX594/TG6006’s potential in hepatocellular carcinoma (HCC). The high response rates and evidence of the specific activity of JX594 on tumour cells in the HEP016 study suggest intravenous administration of JX594 should lead to a survival benefit. If the full data from this trial and three others are positive, a Phase III study with JX594 could start next year. Important news on TG4040 in HCV infections and TG4010 in non-small cell lung cancer (NSCLC) also could offer catalysts for a re-rating of Transgene’s shares.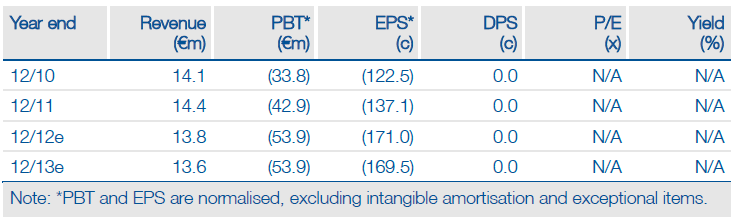
Clear anti-tumour activity seen in new Phase II study
Interim data from the Phase II HEP016 trial in Asian patients with the oncolytic virus JX594 and sorafenib in advanced HCC (20 refractory to sorafenib and five treatment-naive patients) showed that 13 of 21 evaluable patients (62%) had disease control as measured by mRECIST. Tumour biopsies from four patients indicated that intravenous JX594 selectively targeted tumour cells without affecting normal liver cells. Also 15 of 20 evaluable patients (75%) had an objective response by Choi criteria.
JX594 could enter Phase III in H213
More data for the HEP016 trial are due around the end of 2012 and results from three other trials with JX594 are expected in mid-2013: Phase II TRAVERSE study in HCC (n=120); Phase II HEP021 study in HCC (n=c 20); and Phase I/II CRC019 trial in colorectal cancer (n= c 50). If the results of these trials are similar to those from past trials, a Phase III study in first- or second-line HCC could begin in H213.
Important news on TG4040 and TG4010 expected
In November 2012, the final SVR data from the Phase II trial with TG4040 in HCV will be presented at AASLD and the appointment of Dr. Nathalie Adda as CMO (previously at Vertex and Gilead) increases the prospect of a Phase II trial with TG4040 and GS7977. In H113, PFS data from the initial stage of the Phase IIb/III trial with TG4010 in NSCLC should be published. Within 90 days of receiving the results, Novartis has to decide on whether to exercise its option to acquire the rights to TG4010.
Valuation: DCF valuation of €575m
We have increased our Transgene valuation by €21m to €575m because of changes to our estimates following H112 results (adj EPS loss increased by 5.0c in FY12 and reduced by 4.9c in FY13) and to discount factors due to the progression of time. The company had €121m in cash at H112 and should be able to operate into H214.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Transgene Clinical Trial Data Provides Further Positive Results
Published 09/24/2012, 08:03 AM
Updated 07/09/2023, 06:31 AM
Transgene Clinical Trial Data Provides Further Positive Results
JX594 continues to impress
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.