Topotarget’s (OMX: TOPO) key asset belinostat remains on track to be filed with the FDA for the treatment of peripheral T-cell lymphoma (PTCL) in mid-2013 and be approved in H114. Data from the BELIEF trial shows its efficacy is at least similar to that seen with two other drugs approved in recent years in PTCL and it has a better safety profile. The successful filing of the NDA will lead to Topotarget receiving $10m in cash and one million shares from its partner Spectrum. This will enable Topotarget to develop the product in other orphan indications. We increase our valuation to DKK1,285m.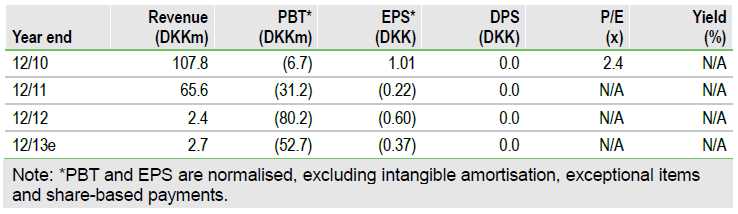
Belinostat efficacy at least similar to competitors…
The objective response rate (ORR) observed in the pivotal BELIEF study (n=129) with belinostat in PTCL was between 25% and 27% (detailed data potentially at ASCO meeting in June). This ORR is in line with that seen in the pivotal PTCL trials with romidepsin (Istodax, 25.4%) and pralatrexate (Folotyn, 26.6%), and meets the requirements of the special protocol assessment (SPA). Also the final data could suggest belinostat is more efficacious than its peers as the BELIEF trial included more poorly patients (with low platelet levels) than comparable trials.
... and safety profile is better
The safety data from the BELIEF trial are similar to what was seen in other belinostat trials, and show that the drug has a better safety profile than romidepsin and pralatrexate. Fewer haematological adverse events were seen than in comparable trials with the other drugs. Belinostat was even well tolerated in patients with low platelets, who are unable to endure treatment with romidepsin and pralatrexate.
Topotarget planning an orphan drug strategy
Topotarget will receive $10m in cash and one million Spectrum shares (currently worth $8m) if the FDA accepts the filing of belinostat for PTCL, which should happen in Q313. This will enable Topotarget to develop belinostat for PTCL and other orphan indications in Europe, without being dependent on Spectrum or potential partners to conduct the clinical trials or market the product.
Valuation: DCF valuation of DKK1,285m
We have increased our valuation by DKK142m to DKK1,285m, after a detailed review of our valuation (including changing potential indications for belinostat and the DKK26.5m equity raise in March 2013). Topotarget should have sufficient cash to operate into FY14 after the share issuance, by which time it could have received the milestone payments for the NDA filing of belinostat being accepted by the FDA.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Topotarget: Planning An orphan Drug Strategy
Published 03/26/2013, 07:22 AM
Updated 07/09/2023, 06:31 AM
Topotarget: Planning An orphan Drug Strategy
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.