The BELIEF pivotal study with Topotarget’s belinostat has met its primary endpoint with more than 20% of patients responding to treatment, causing the share price to rise 135%. Full data should be reported in Q412, which will indicate how belinostat compares to other treatments for peripheral T-cell lymphoma (PTCL) that have been approved in recent years. Topotarget remains on track to receive milestones of $10m in cash and one million Spectrum shares in H213 and for belinostat to be launched in 2014. We have increased our valuation from DKK909m to DKK1,118m.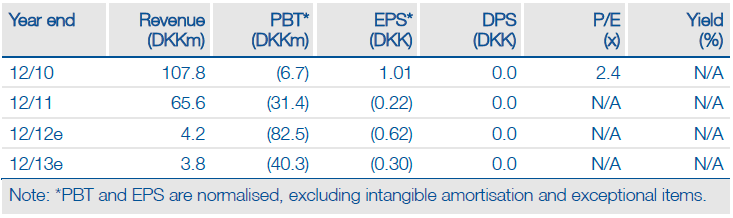
Primary Endpoint Met In BELIEF Study
Topotarget has announced that the pivotal Phase II BELIEF study in PTCL has achieved the primary endpoint of >20% overall response rate (ORR). The full data from the trial is expected to be reported in Q412, with details of complete/partial responders and safety profile.
Milestone Payments Expected In H213
Spectrum Pharmaceuticals should file the NDA with the FDA using the data from the trial, which should lead to Topotarget receiving a $10m cash milestone and one million Spectrum shares (currently worth $12.5m) in H213. Although the BELIEF study has met the predefined endpoint of >20% ORR agreed with the FDA in a special protocol assessment (SPA), the FDA will reassess whether the benefit/risk profile of belinostat is sufficient for approval. However, belinostat remains on track to be launched in 2014.
Full Data Still Needed To Assess Potential Of Belinostat
The full data from the trial are needed to assess how belinostat compares to other treatments for PTCL that have been approved in recent years and this could be another catalyst for Topotarget’s shares. The ORR of pralatrexate (Folotyn, marketed by Allos Therapeutics, which has just been acquired by Spectrum, sales of $50m in FY11) was 27% and of romidepsin (Istodax, a pan-histone deacetylase inhibitor in the same class as belinostat) was 25% in their pivotal trials. So far, clinical trials suggest that belinostat has a more favourable safety profile than these drugs. So, if belinostat’s efficacy is comparable, the product should be competitive in the PTCL market.
Valuation: DCF Valuation Of DKK1,118m
We have increased our valuation by DKK209m to DKK1,118m after increasing the likelihood of belinostat achieving peak sales of $72m from 60% to 75%. We will review our valuation further once the full data from the BELIEF trial are released.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Topotarget Clinical Trial Announcement
Published 09/28/2012, 08:01 AM
Updated 07/09/2023, 06:31 AM
Topotarget Clinical Trial Announcement
The Power Of BELIEF...
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.