TiGenix (TIG.BR) has made good progress in developing its EU stem cell business. The Dutch healthcare system now fully reimburses ChondroCelect enabling TiGenix to invest in marketing and product support into Holland. The crucial Cx601 study in perianal fistulas started on 10 July as expected. The Cx621 safety study showed that intralymphatic groin injection of adipose stem cells was safe and well tolerated. A US partner on Cx601 may be agreed in H212-H113.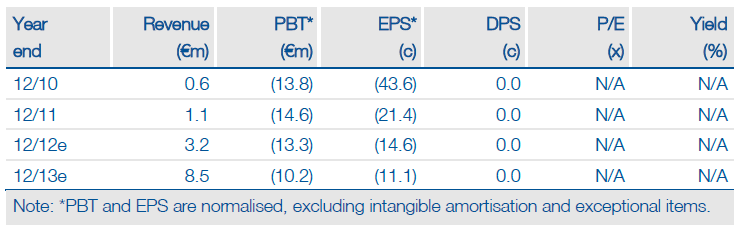
Dutch reimbursement important step forward
ChondroCelect produces higher-quality cartilage than other procedures with five-year clinical superiority over microfracture. The Dutch National Health Authority allowed full reimbursement for ChondroCelect implants done from January 2012. Other countries will presumably come to the same conclusions but the timing of these is uncertain; at least one more may do so in 2012. The ATMP regulations might drive cheaper cell-based products from the EU market in 2013, but this is not certain. Edison expects ChondroCelect implant sales of 180 in FY12 and over 450 in FY13.
Cx601 enters pivotal study, Cx621 procedure safe
Cx601 is injected directly into Crohn’s perianal fistula tracts to cut inflammation and aid healing. Phase III recruitment started on 10 July. About 120,000 patients in the EU and US have fistulas; 60% respond poorly to therapy. With direct EU sales from 2016 plus an anticipated US partner, Cx601 could be highly lucrative. The Cx621 safety study on inguinal (groin) lymph-node injection reported on 2 July. None of the 10 patients experienced any side effects and the procedure was well tolerated. Injection was guided by echographic imaging, but, in practice, administration can be done direct by experienced physicians. Intralymphatic injection could enable lower doses (5m cells) and more effective therapy for autoimmune diseases.
Valuation: Break-even on ChondroCelect, value on Cx601
Edison assumes profitability by 2015 if over 1,675 ChondroCelect implants are sold at about €18k each. Adding direct Cx601 EU sales from 2016 (55% probability) plus sales by the anticipated US partner from 2017 (45% probability) gives an indicative value on rDCF (at 12.5%) of €2.33 per share or €205m. An alternative 1,000 implant sales scenario indicates €1.70 per share. TiGenix is funded into 2013, assuming ChondroCelect unit sales of 200-250 in 2012, but may need up to €4m from non-dilutive funding to realise Cx611 (autoimmune cellular therapy) clinical goals and move further funding into 2014.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
TiGenix Clinical Trial: On Track
Published 07/17/2012, 12:06 AM
Updated 07/09/2023, 06:31 AM
TiGenix Clinical Trial: On Track
Fistula relief on track
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.