Tekmira Pharmaceuticals (NASDAQ:TKMR) is a biopharmaceutical company based in Vancouver, Canada. Tekmira prides itself on creating drugs to combat cancers, viral infections, and metabolic diseases in orphan markets. Their most well-known developing drug is TKM-Ebola, which is in Phase 1 of FDA testing to treat the rapidly spreading Ebola virus in West Africa. The pharmaceutical company released its third quarter earnings on November 6th, falling short of earnings estimates.
Tekmira’s Q3 report posted net losses of -$0.39 per share, a 5% year-over-year increase from -$0.41 per share. This fell short of the consensus estimate of -$0.32 per share. Revenue for the third quarter was $4.4 million, an 18.5% year-over-year increase from $3.0 million, and beating the consensus estimate of $3.71 million.
President and CEO Dr. Mark Murray remained altruistic, noting “Our goal this quarter was to continue the advancement of our product development programs, reflecting the Company's focus on developing novel RNAi-based therapeutics.” In regards to the Ebola outbreak, Dr. Murray continued, “We have designed and initiated production of a modified RNAi therapeutic directed against the Guinea variant of the Ebola virus” Dr. Murray announced that Tekmira is being reimbursed by the Department of Defense for all costs incurred for the development of TKM-Ebola, which “shows a commitment to the continued development of anti-Ebola therapeutics”
Tekmira, in collaboration with the U.S. Army, has been working on an Ebola drug therapy, called TKM-Ebola, to combat the devastating virus is West Africa. In March 2014, the drug was granted “Fast Track” access to the FDA as the need for a widespread Ebola drug became urgent. Currently, the drug is on a partial clinical hold. This status allows the drug to be used in people who have Ebola but requires Tekmira to continue collecting and providing more information about the mechanism of the drug.
As CEO Dr. Murray mentioned, Tekmira is working on a modified version of the Ebola drug. Because Ebola mutates as it spreads, Tekmira has created a variant of TKM. A press release dated October 21st, 2014 confirms that Tekmira “has completed the design of a modified RNAi therapeutic specifically targeting this viral variant, now termed 'Ebola-Guinea.” This drug variant targets the strain of Ebola most prevalent in West Africa. Doses of the experimental drug will be available in early December.
Shares of Tekmira opened at $16.70 on Monday, November 10th. The company has a 1-year high of $31.48 and a 1-year low of $7.17. The daily moving average is $16.36 and the 50-day moving average is $23.14. The market cap for Tekmira is $410.27 million and its P/E ratio is not applicable.
On November 10th, Glaucus Research Group rated Tekmira a Strong Sell on Seeking Alpha with a price target of $7-$10. The research group cited many concerns, including safety trepidations for TKM-Ebola. Glaucus believes the drug is going to fail FDA approval because the “technology in unproven.” Additionally, doctors are reluctant to administer the drug because they believe it is unsafe and prefer ZMapp; another experimental drug. Glaucus concluded, “Tekmira is hugely overvalued and its share price is poised to collapse.” Glaucus Research Group has rated two stocks, earning a 50% success rate.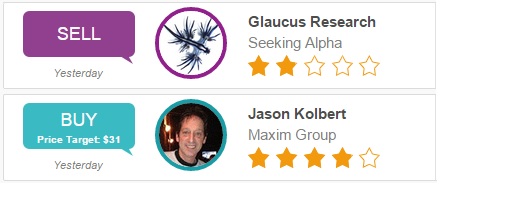
Separately, Jason Kolbert of Maxin Group maintained a Buy rating for Tekmira with a $31 price target on November 10th. Kolbert cited the Department of Defense’s $7 million reimbursement for TKM-Ebola, “which should be available in December 2014.” He added, “Several Ebola-infected patients (primarily in the US) have been treated as part of emergency use granted by the FDA, though success or failure was not disclosed, and any success would not constitute a clinical trial. We expect TKM-EBOLA to be in clinical trials in 2015.” Kolbert has made 142 ratings since 2009, earning a +5.2% average return per recommendation.
On average, the top analyst consensus for Tekmira is Moderate Buy.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Tekmira Pharmaceuticals Gets Mixed Ratings After Ebola Drug Updates
Published 11/11/2014, 10:53 AM
Updated 07/09/2023, 06:32 AM
Tekmira Pharmaceuticals Gets Mixed Ratings After Ebola Drug Updates
Latest comments
Loading next article…
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.
