Synta (SNTA) is to focus in future on combination studies for ganetespib, as it launches its pivotal Phase III GALAXY-2 trial of docetaxel±ganetespib in second-line non-small cell lung cancer (NSCLC). The change is likely to mean an early end to the CHIARA study, which examines ganetespib as monotherapy in ALK+ NSCLC. The shift in strategy will also allow it to focus resources on the large GALAXY-2 study, which is now central to the investment case. The publication of the full ITT data from GALAXY-2’s predecessor, GALAXY-1, which are due in June, is the next major catalyst. Our rNPV of Synta is $790m or $11.44/share (basic) or $10.59/share (diluted).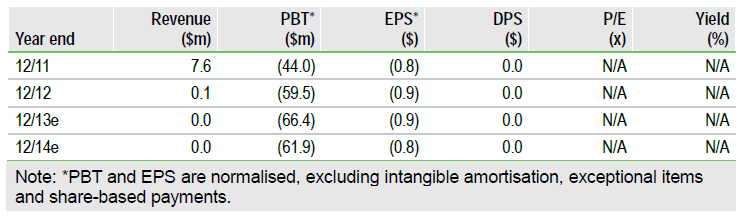
New combo strategy as GALAXY-2 gets underway
Synta is to shift its future development strategy for ganetespib further away from monotherapy towards combination studies, as it launches the large pivotal GALAXY-2 trial of ganetespib in combination with docetaxel in second-line NSCLC. Synta aims to enrol 500 patients NSCLC patients after failure on first-line therapy, excluding fast progressors (patients who have reached second-line therapy within six months of diagnosis). The design was based on interim results of the GALAXY-1 study, the top-line ITT results of which are likely to be presented in June.
Future of CHIARA study more ascuro
Synta intends to review the CHIARA Phase II study of ganetespib as monotherapy in crizotinib-naïve ALK-positive NSCLC, but its change in strategy suggests this study is likely to be closed. An investigator-sponsored study is examining ganetespib in combination with crizotinib, in patients failing on crizotinib. Such a move, if confirmed, would also reflect the changing landscape in the ALK+ area, where competitors are focussed on combinations with direct ALK inhibitors.
Enchant – new ganetespib/paclitaxel arm
The ENCHANT Phase II study in metastatic breast cancer is also to be amended to add a combination arm of ganetespib + paclitaxel alongside the originally planned genetespib monotherpy arm in this study.
Valuation: $10.59 per share
We have made very minor adjustments to our valuation of Synta, which is now total value of $790m, equivalent to $11.44/share (basic) or $10.59/share (fully diluted). The valuation of ganetesib is unchanged at $743m and uses a conservative 50% (for a Phase III asset) probability in NSCLC. We intend to review this after publication of the GALAXY-1 OS data in June.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Synta Pharmaceuticals: New Combo Strategy As GALAXY-2 Gets Underway
Published 04/04/2013, 08:43 AM
Updated 07/09/2023, 06:31 AM
Synta Pharmaceuticals: New Combo Strategy As GALAXY-2 Gets Underway
Latest comments
Loading next article…
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.