In an unfortunate turn, SymBio Pharmaceuticals Ltd (T:4582) has run into some quality control issues with its shipments of Treakisym from Astellas. During Q219, the company found an unacceptable level of ‘foreign substance or visual defects’ in the shipments of drugs, which had to be returned. The company revised its sales estimates down by a third (to ¥3.0bn from ¥4.5bn) due to the expected short supply.
2019 guidance: Modest increase in operating losses
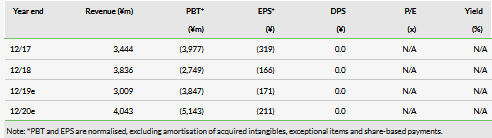
Although the company expects the supply issue to affect revenue by ¥1.4bn, the impact on the bottom line is expected to be more moderate. The company guided to a net loss of ¥3.8bn, only slightly increased from previous estimates of ¥3.6bn. The company did not provide in depth detail except that it expects SG&A cost to be approximately ¥300m less than previous estimates.
Revenue unlikely to be fully recouped
SymBio did state that it expects Astellas to ship replacement product by Q120. However, this supply issue comes at a complicated time for the company. The company’s marketing agreement with Eisai expires in 2020, and SymBio has plans to establish its own salesforce to take over in 2021. However, it expects Eisai to wind down its inventory in 2020, which will have a negative impact on sales. SymBio is currently assessing how 2020 revenue will be affected, and has not issued new guidance, but we do not expect the lost revenue from 2019 to be entirely recouped.
Eyes on the lookout for DLBCL results
We expect the top-line results from the ongoing Phase III registration trial of Treakisym for diffuse large B-Cell lymphoma (DLBCL) in H219, which will be the biggest near-term inflection point for the company. DLBCL is the most common form of non-Hodgkin lymphoma; the drug is already approved for indolent forms of it in the US and Japan. DLBCL is a more aggressive form of the disease and the most common (45%) in Japan. We estimate that adding the indication would approximately double the addressable market for the drug.
Valuation: Increased to ¥28.9bn ($255m)
We have increased our valuation of SymBio to ¥28.9bn ($255m), from ¥26.8bn ($237m), although it is lower on a per share basis (reverse split adjusted): ¥1,185 ($10.49) from ¥1,236 ($10.94). The increase is driven by rolling forward our NPVs and higher net cash (¥6.1bn) following a ¥2.5bn equity raise, and offset by lost revenue due to the Treakisym supply issue.
Business description
SymBio Pharmaceuticals is a Japanese specialty pharma company with a focus on oncology and haematology. The Treakisym powder formulation was in-licensed from Astellas in 2005; liquid Treakisym was in-licensed from Eagle Pharmaceuticals in 2017. Rigosertib was in-licensed from Onconova.
Treakisym supply affected by bad batches
SymBio reported its H119 financial results on 7 August 2019. As part of this release the company announced a change in its mid-range plan (previously announced in February 2019), which included downward revision of the company’s revenue primarily in 2019. The cause of the downward adjustment was directly attributed to supply issues with the company’s product Treakisym (bendamustine) it sources from Astellas. A batch that the company received in Q219 was rejected by its quality control personnel because it appeared to be contaminated. The company believes that the replacement shipment may come as late as Q120, and therefore the ability to fill orders will likely be affected in 2019. It lowered its sales guidance by approximately a third to ¥3.092bn from ¥4.465bn.
The company has not released any additional changes to the mid-range plan such as changes to 2020 guidance and beyond, but it said that it is reviewing the potential impact on these years. We believe that it is unlikely that the company will recoup all of the lost revenue from the quality control incident in 2020. The product is currently marketed in Japan by Eisai, but the agreement expires in 2020. Eisai is expected to wind down its inventory, which SymBio cited as the reason for low sales guidance for 2020. The shipment of replacement product should increase inventory that needs to be run down, but we suspect that marketing efforts will also be reduced. Additionally, it would be unlikely for the company to recoup all its losses in future quarters simply because of the severity of the diseases being treated and the fact that patients cannot wait indefinitely for treatment.
Although revenue guidance was heavily penalized, the company’s guidance on earnings was less affected. It only estimated a ¥193m negative impact on operating losses for 2019 (¥3.6bn from ¥3.8bn), and although it did not break out all the details this was at least partially driven by lower SG&A expenses.
Looking forward: Focus now on DLBCL results
The company is current in a Phase III study of Treakisym in diffuse large B-cell lymphoma (DLBCL), and announced that it completed enrolment in April 2019. We expect it to provide top-line results in H219, and for this to be a major value inflection point for the company, as it is our second largest value driver (beside other sales for Treakisym). The primary endpoint of the study will be overall response rate, with progression free survival and overall survival as secondary endpoints. Although bendamustine is not approved explicitly for DLBCL in the US (although it is approved for indolent non-Hodgkin lymphoma), it has been demonstrated to have activity when combined with Rituxan (rituximab).1 We estimate a target market of 11,200 second-line DLBCL patients, which is approximately the size of the current addressable market. The company has guided to a Q220 Japanese NDA filing and we expect an approval decision in H221.
Valuation
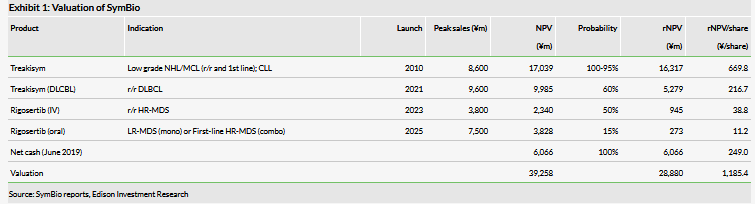
We have increased our valuation of SymBio to ¥28.9bn ($255m) from ¥26.8bn ($237m), although it is lower on a per share basis (reverse split adjusted), due to an increase in shares outstanding: ¥1,185 ($10.49) from ¥1,236 ($10.94). This increase is driven by rolling forward our NPVs and higher net cash (¥6.1bn), and is offset by the impact of the Treakisym supply issues. We expect to update our estimates with the release of the top-line DLBCL data in H219.
Financials
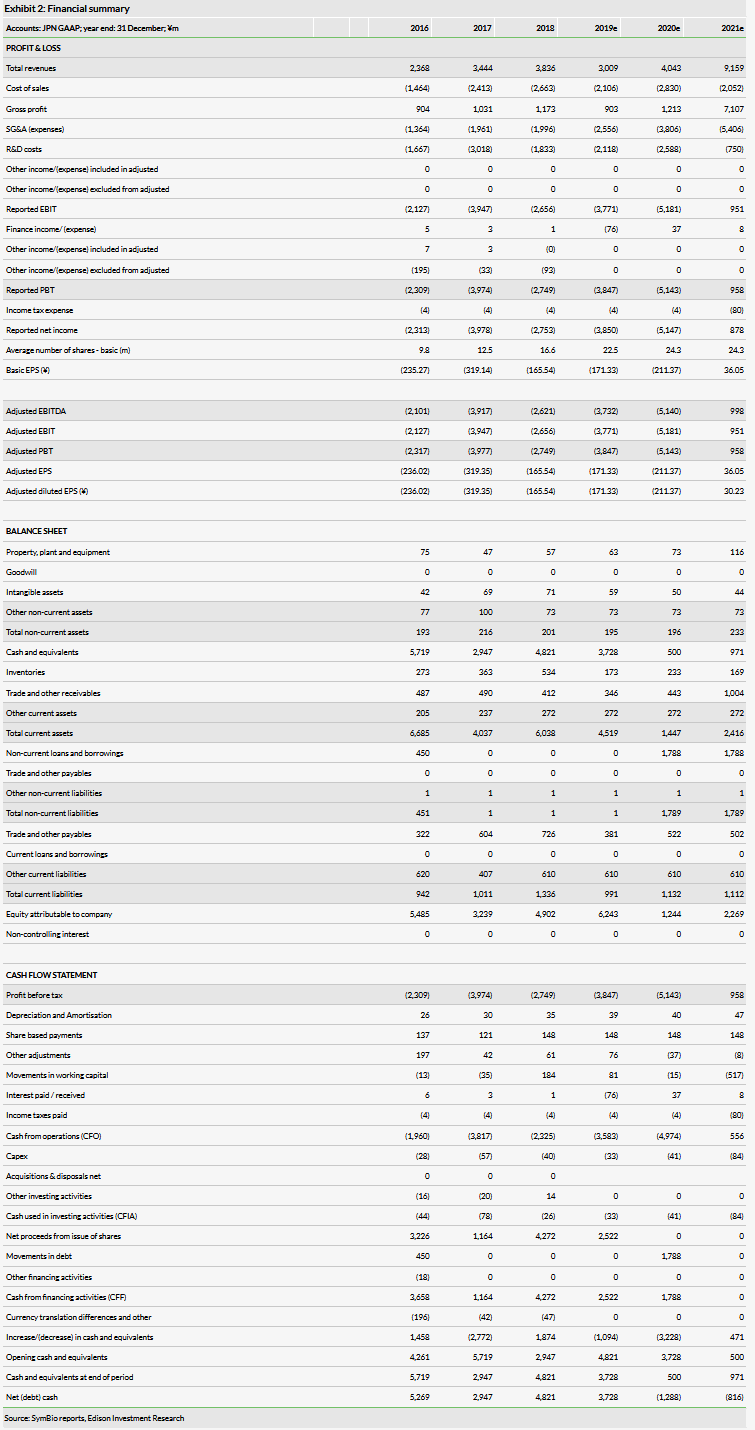
Despite the adjustments to guidance for the year, the company’s results reported for H119 were largely within our expectations. Revenue was ¥2.0bn, which is roughly flat on a year-on-year basis, and we expect this to be the highest sales for the product until market rights are transferred back to SymBio. We currently forecast revenue of ¥4.0bn for 2020, up from ¥3.2bn due to recovering approximately half of the sales lost in 2019 due to the supply issues. However, this is offset by a slight increase to our SG&A estimates (¥3.8bn from ¥3.7bn) to bring them in line with current trends. Otherwise our forecasts remain unchanged. The company ended the period with ¥6.1bn in cash following the raise of ¥2.5bn from share acquisition rights. We expect the company to need ¥1.8m in additional financing in 2020 (which we include as illustrative debt) to reach profitability in 2021.