Sucampo’s (SCMP) launch of glaucoma drug Rescula onto the US market is its first self-commercialisation effort. Careful product positioning and a targeted commercial campaign, emphasising Rescula’s novel mode of action, should give the drug a competitive foothold in a highly competitive market. The wholesale acquisition cost (WAC) of $99 per script (30-day eye drop supply) is on a par with some branded prostaglandins (Zioptan) and is higher than our previous price assumption. We raise our peak US sales estimate for Rescula to $90m (previously $65m) and increase our valuation of Sucampo to $335m or $8.00 per share (vs $315m, $7.52 per share).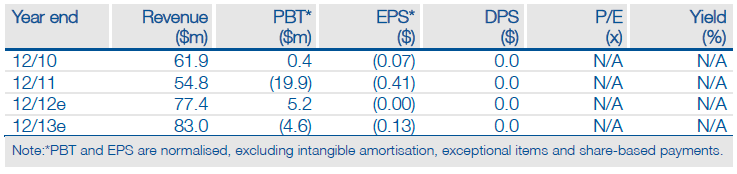
Still room in the market
Prostaglandin analogues – Xalatan (now generic), Lumigan, Travatan – are once-daily eye drops used most commonly as the first line of therapy for lowering intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension. However, tolerability, particularly hyperaemia (red eye), is an issue with prostaglandins, leaving a decent portion (c 50%) of the glaucoma market for alternatives, currently occupied by beta-blockers, alpha agonists and carbonic anhydrase inhibitors (CAIs).
Positioning and label is key
Rescula, with its prostone-based, ion channel (BK and ClC-2) activating technology, offers an alternative mechanism of action to prostaglandins and the key second-line therapies. Although Rescula can be used as a first-line agent, Sucampo will primarily focus on positioning the drug as a safe and more convenient second-line option for patients intolerant, or failing to respond, to prostaglandins.
Better chance this time
Rescula, a twice-daily eye drop, was approved by the FDA in 2000 and marketed in the US (2000-2002) by previous licensee Ciba Vision, but uptake was restricted by its classification as a prostaglandin, bracketed as a ‘me too’ to the fast-growing brands. The recent FDA approval of Rescula’s revised label, not classified as a prostaglandin, was vital, and clearer positioning of the product, supported by 40 reps directly targeting 50% of specialist prescribers, gives Rescula a stronger competitive position.
Valuation: Increased to $335m, $8.00 per share
We increase our valuation of Sucampo to $335m (vs $315m) or $8.00 per share ($7.52), mainly as a result of raising our peak US sales estimate for Rescula to $90m (vs $65m) – Rescula now contributes $110m, or $2.62 per share, to our total DCF-based valuation. We note that peak sales could be considerably higher (up to $150m), but await prescription trends, especially beyond the initial phase of heavy sampling.
To Read the Entire Report Please Click on the pdf File Below.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Sucampo Pharmaceuticals Launches Rescula In The U.S.
Published 03/05/2013, 06:56 AM
Updated 07/09/2023, 06:31 AM
Sucampo Pharmaceuticals Launches Rescula In The U.S.
Competitive footing for Rescula
Latest comments
Loading next article…
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.