The FDA’s acceptance of the Oralair filing has re-awakened hopes that Stallergenes is still in the race to launch the first allergy immunotherapy tablet in the US. Progress with the tablets in EU, US and Japan in various indications is likely to be a key value driver for the shares, with first potential US Oralair launch in Q114, and continuation of the clinical development plan of the dust mite Actair tablet during 2013.
U.S. Hopes Re-Awaken With Filing Acceptance
Stallergenes disclosed the FDA’s acceptance of the December Oralair filing last week. The acceptance suggests Stallergenes may have addressed any major concerns or questions raised by the FDA. At its 19 February investor day Stallergenes confirmed that is exploring all options in the US, including commercialisation alone or with a partner. Management believes the US market would need c 100 sales reps to target 10,000 allergists and ENTs that currently treat c 2.8m patients with allergy shots. Stallergenes does not intend to compete directly with allergy shots, but instead will target patients that refuse allergy shots or drop-out of treatment within one year.
Just Ahead Of Merck’s Filing In The U.S. Race
Stallergenes’ December Oralair filing for severe allergic rhinitis does put it a few months ahead of competitor ALK-Abello’s filing, announced 1 February, partnered with Merck & Co. in the US. Although there have been no head-to-head studies of Oralair versus GRAZAX, reported efficacy from EU trials is broadly similar. Oralair has a shorter 5-6 month treatment duration and contains extracts from five grasses, whereas GRAZAX is taken throughout the year and contains a single grass extract. GRAZAX has data in nearly 2,000 US patients from two Phase III trials, the latest trial in 1,500 patients having been completed following FDA feedback. Oralair has Phase III data in c 500 patients from a single US-based trial.
2012 Growth Despite Challenging Markets
FY12 sales grew +3.3% despite challenging EU markets. This was driven by new international markets and introductions of Oralair (launched in France in December). Geographic expansion is one of the key strategies to drive future growth.
Valuation Will Be Driven By Tablets
Stallergenes’ €82m end-June net cash implies an EV of c €590m. This seems broadly underpinned by its growing, profitable business with €243m FY12 sales. Allergy tablet newsflow, particularly in the US, is likely to be the key value driver.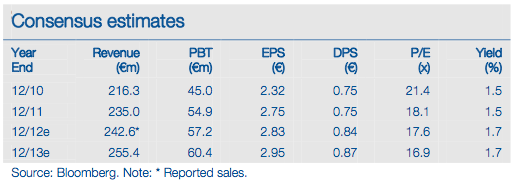
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Stallergenes: A Quick Look
Published 02/25/2013, 02:03 PM
Updated 07/09/2023, 06:31 AM
Stallergenes: A Quick Look
Investment Summary: U.S. Hopes Re-Awaken
3rd party Ad. Not an offer or recommendation by Investing.com. See disclosure here or
remove ads
.
Latest comments
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2024 - Fusion Media Limited. All Rights Reserved.