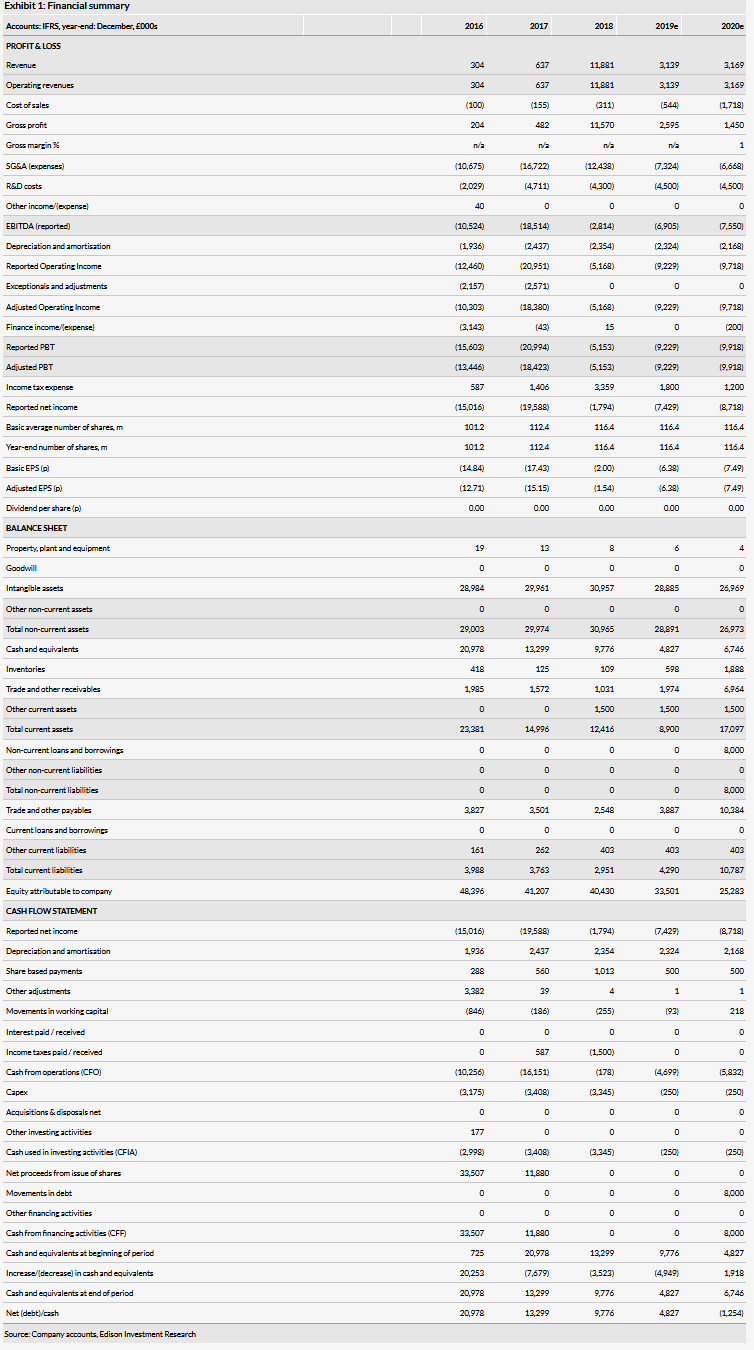
Shield Therapeutics (LON:STXS)’ primary asset, Feraccru, has been approved by the FDA for the treatment of iron deficiency in patients with any underlying cause – the broadest possible label. It will be marketed in the US as Accrufer. This is upside to our previous assumptions and increases our peak sales potential to c$420m (vs c $250m previously). Successfully commercializing Feraccru/Accrufer through partners is now key to Shield realizing its value. We expect Shield to out-license US rights during the next 18 months. An upfront licensing payment would extend Shield’s cash reach beyond our current forecast of H220. We now value Shield at £273m.
Broadest label possible opens up US market
There remains a significant need globally for a tolerable oral iron therapy and this approval has now de-risked the opportunity in the US. Due to a broader than predicted label, we increase our peak sales forecasts for the US to c $420m vs $250m previously. We retain our peak sales forecast of c €130m for the EU5 states, as covered by Norgine. With positive data in hand from the AEGIS-H2H study, demonstrating non-inferiority to the market-leading iv iron (Vifor’s Ferinject 2018 sales of c $0.9bn), Accrufer could quickly generate significant market share in both the US and EU.
Partner execution key to sustaining top-line growth
Out-licensing Accrufer in the US is now the next step to commercialization. Furthermore, an upfront licensing payment would alleviate the need to raise additional capital by mid-2020. With the approved broad label of Accrufer, we anticipate that Shield will be able to negotiate deal terms in line or better than those achieved with Norgine in Europe. We forecast sustainable profitability from 2022, with gross margins nearing c 50–60% in the long term. With Norgine now actively marketing Feraccru in Germany and the UK, we anticipate an uptick in sales during 2019. Further launches in additional European markets during 2020 (and potentially in the US contingent on a deal) will aid revenue growth (Shield receives royalties on sales).
Valuation: £273m or 231p/share
Our revised valuation of Shield at £273m or 231p/share vs £177m or 152p/share (derived from an rNPV model) reflects the removal of regulatory risk from our valuation and the increased market opportunity as a result of the broad US label. We have also updated for FX and rolling forward our model in time. Uncertainty about the eventual outcomes from patent challenges raised by Teva Pharmaceuticals still represents c 35% downside to our base case (82p/share).
Business description
Shield Therapeutics is a commercial-stage pharmaceutical company. Its proprietary product, Feraccru, is approved by the EMA and FDA for the treatment of iron deficiency. Feraccru is currently marketed through partners Norgine, AOP Orphan and Ewopharma.