Puma Biotechnology, Inc. (NASDAQ:PBYI) announced that the FDA has approved a labeling supplement for its breast cancer drug Nerlynx (neratinib) to include safety information based on interim results from the phase II CONTROL study.
The study evaluated the antidiarrheal prophylaxis or dose escalation in the reduction of Nerlynx-associated diarrhea that had a primary endpoint of the incidence of grade III or higher diarrhea.
Data from the CONTROL study showed that the addition of prophylactic treatment with Imodium (loperamide) + budesonide reduced the rate of discontinuation due to Nerlynx-related diarrhea to 11% in HER2-positive early stage breast cancer patients compared with a discontinuation rate of 18% regarding loperamide alone.
Moreover, patients who were treated with the combo of loperamide plus budesonide, the incidence of grade III diarrhea was 28% compared with 32% for patients who received loperamide only.
We remind investors that Nerlynx’s sales have been hurt this year due to a higher number of patients stopping treatment with Nerlynx due to diarrhea and other side effects. On second-quarter 2019 conference call, the company stated that the current label for Nerlynx only includes data from the sole loperamide arm of CONTROL study in which discontinuations due to diarrhea or other side effects were 44.5%.
It is expected that the FDA approval for the inclusion of this new data from the CONTROL study in the label will increase awareness of the loperamide plus budesonide combination, which could help lower discontinuations.
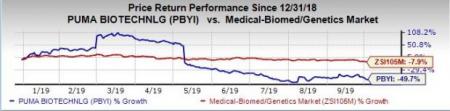
Shares of Puma Biotech were up almost 4.2% on Thursday following the above news on Oct 2. However, the stock has plunged 49.7% so far this year, wider than the industry’s decrease of 7.9%.
Nerlynx is indicated for extended adjuvant treatment of HER2-positive early stage breast cancer in patients, previously treated with Roche's (OTC:RHHBY) Herceptin-based adjuvant therapy. The drug was approved in the EU for the same indication last September.
Meanwhile, last month, the FDA approved a supplemental new drug application (sNDA) for Nerlynx in combination with Roche's Xeloda to treat HER2-positive metastatic breast cancer in patients who have failed two or more prior lines of treatments. The regulatory body will announce its decision related to the sNDA in April 2020.
This sNDA was based on data from the phase III NALA study, which assessed the combination of Nerlynx + Xeloda compared to Xeloda plus Novartis' (NYSE:NVS) Tykerb (lapatinib) in the above-mentioned advanced breast cancer patient population.
Notably, Nerlynx is the only marketed product in Puma Biotech's portfolio. The drug generated sales of $99.4 million in the first half of 2019, reflecting an increase of 14.5% year over year. Potential approval of label expansions will further boost the drug's sales.
Zacks Rank & Stock to Consider
Puma Biotech currently carries a Zacks Rank #3 (Hold). A better-ranked stock in the healthcare sector is Incyte Corporation (NASDAQ:INCY) , which sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Incyte’s earnings estimates have been revised 4.9% upward for 2019 and 4.6% for 2020 over the past 60 days. The stock has rallied 17.7% year to date.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple (NASDAQ:AAPL) sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 27 billion devices in just 3 years, creating a $1.7 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 6 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2020.
Click here for the 6 trades >>
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
Novartis AG (NVS): Free Stock Analysis Report
Incyte Corporation (INCY): Free Stock Analysis Report
Puma Biotechnology, Inc. (PBYI): Free Stock Analysis Report
Original post
Zacks Investment Research
