Valuation
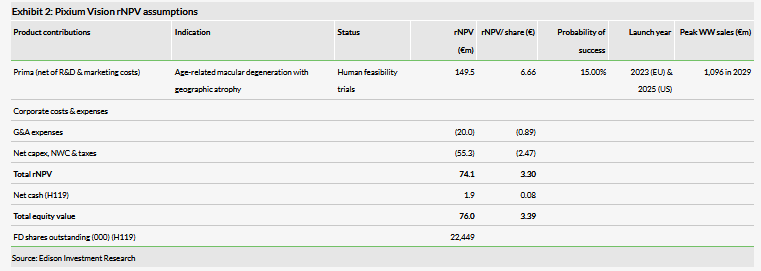
We continue to value Pixium using an rNPV approach, employing a 12.5% cost of capital. Our valuation is based solely on the Prima opportunity in Dry-AMD. We continue to apply a probability of success estimate for Prima-ARMD in our model of 15% and we assume a forex rate, for US sales, of $1.12/€.
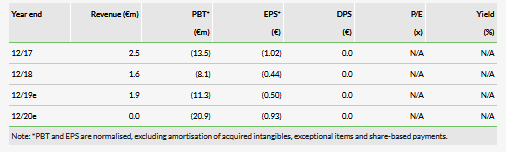
After pushing our EU commercialisation timelines by about six months and our US market launch assumptions by 12 months, and rolling forward our estimates, we now obtain a pipeline rNPV (enterprise value, excluding net cash) of €74.1m, versus €91.7m previously. After including €1.9m in net cash at 30 June 2019, we obtain an equity valuation of c €76.0m, or €3.39 per share (compared to €4.49 previously).
Pixium Vision SA (PA:PIX) recently reported H119 financial results, with operating cash flows consistent with expectations. Importantly, the company announced improvements to the Prima Bionic Vision System (BVS), particularly with respect to the augmented reality (AR) glasses worn by patients, termed Prima 2. It plans to conduct feasibility testing on the second-generation system prior to commencing EU pivotal studies, which are now planned to start in H120 (vs H219 previously). This may de-risk the system prior to starting pivotal trials, but pushes back our EU and US commercialisation projections to 2023 and 2025, respectively, versus our prior forecasts of H222 and 2024, respectively. We now obtain a pipeline rNPV (including net cash) of €76.0m, vs €99.5m previously.
12-month EU feasibility data confirms safety
Pixium recently reported positive 12-month data on its initial (ongoing) five-patient EU Prima feasibility study in patients with advanced dry-AMD. This data is consistent with the six-month results reported in January, which showed that the patients, who had no central visual activity at enrolment, were all able to perceive light perception through the Prima system. Safety data is also favourable.
Second-generation Prima aims to improve function
Pixium plans to shortly announce more detail on its planned upgrades to the Prima system, which are designed in part to enable implanted dry-AMD patients to combine both prosthetic and natural residual vision. The second-generation system is based on the existing 378-electrode implant chip, but uses a new version of the augmented-reality glasses (Prima 2), which we expect to incorporate more advanced image processing and artificial intelligence functionality and is designed to enhance the visual experience for patients. Pixium plans to pursue feasibility testing on the upgraded system (employing Prima 2 glasses) in the five patients already enrolled and implanted in the existing EU feasibility study, and in five US patients as well. Pixium's current strategy assumes an EU pivotal study to start in H120 but the firm is pursuing several approaches to potentially accelerate development and it has ongoing discussions with the EU and US authorities.
Valuation: Lowered to €76m on pivotal trial delay
We believe Pixium’s cash on hand should be sufficient for it to maintain its operations into Q220, and we estimate that Pixium will raise €83m through 2021 to fund Prima development. We model these as debt financing. Given our updated launch timing estimates and after adjusting for €1.9m H119 net cash, we now obtain an equity valuation of €76m, or €3.39/share (vs €4.49/share previously).
Business description
Pixium Vision develops bionic vision systems for patients with severe vision loss. Its lead product, Prima, a wireless sub-retinal implant system designed for dry-AMD, is already in a human feasibility study in Europe and is expected to start implantations in a US feasibility study in H219.
H119 results broadly in line
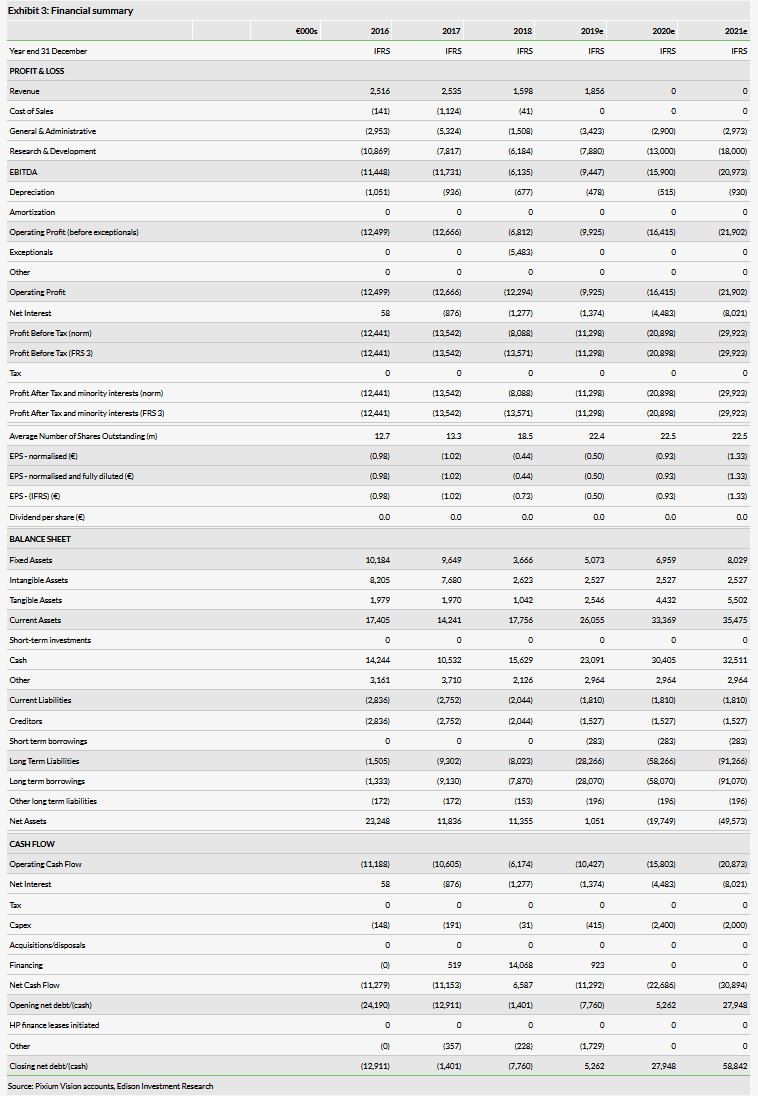
Pixium reported H119 results on 25 July 2019, with operating and net losses mildly above our expectations due to higher than expected expenses. It reported €1.06m in H119 revenue (primarily from subsidies and research tax credits), above our expectation of €0.8m. R&D costs were €3.88m (vs our €3.25m expectation), and the SG&A expense (excluding depreciation) was €2.02m (above our €1.4m forecast). R&D costs were largely attributable to running the ongoing feasibility studies (in both the EU and the US) and with developing and manufacturing the second-generation augmented reality (AR) glasses (‘Prima 2’) to be used with the Prima implant system in future trials, including the planned EU pivotal trial. Reported SG&A costs included a non-recurring non-cash charge of €0.81m reflecting a share-based payment provision relating to the departure of the company’s prior CEO. Excluding this charge, we calculate Pixium’s SG&A costs (excluding depreciation) would have been would been approximately €1.2m.
Altogether, Pixium had a €5.08m operating loss (above our €4.15m estimate), and a €5.47m net loss (€0.25 per share), versus our expectation of a €4.49m net loss. Operating cash flow was a loss of €5.07m, comparable with our forecast of a loss of €5.17m. The company finished H119 with a gross cash position of €10.2m, and after removing gross debt of €8.35m, we calculate a 30 June 2019 net cash position of €1.9m (slightly below our prior H119 net cash estimate of €2.2m).
12-month EU feasibility study data confirm safety profile
Pixium reported on 18 July 2019 positive 12-month data as part of the ongoing five-patient Prima European feasibility study. The 12-month data is consistent with the interim six-month results reported in January, which showed that after implantation and rehabilitation in patients in France with advanced dry-AMD (geographic atrophy, or GA), the Prima system met the primary endpoint, which was the successful elicitation of light perception in the central retinal area and visual field as measured by Octopus perimetry. None of the patients had remaining central visual activity at the time of enrolment. All patients received visual rehabilitation therapy subsequent to Prima implantation, and at 12 months, the company reported that most can identify letters and have some ability to identify sequences of letters, and have demonstrated increasing recognition speed over time. Safety data remained favourable, as there continue to be no device-related serious adverse events. Overall, the results demonstrate feasibility of the photovoltaic restoration of central vision in dry-AMD patients, while maintaining the residual peripheral natural vision.
In parallel with this EU feasibility study, Pixium is undertaking a five-patient US feasibility study, being conducted at the University of Pittsburgh Medical Center and at Bascom Palmer Eye Institute (Miami, Florida). Management expects the first implantations to occur in H219. Pixium believes that 12-month safety and performance data on all five patients will likely be sufficient for US regulators to allow a larger US (pilot) study to be started. We expect that study data from the US feasibility study should be available in 2021 and that recruitment for the US pilot study can potentially also begin in 2021 (vs our prior H220 forecast). The US feasibility study’s primary endpoint will be elicitation of visual perception of the Prima device, while secondary endpoints will include visual acuity (VA), measured by methods such as the Early Treatment Diabetic Retinopathy Study and Freiburg Visual Acuity & Contrast Test scales.
New EU feasibility study planned using Prima 2 glasses
Pixium has continued to work on making improvements to the Prima system and indicated that it expects to announce more detail on such upgrades soon, which are designed in part to enable implanted dry-AMD patients to combine both prosthetic and natural residual (ie peripheral) vision. As a reminder, the Prima system consists of the implanted subretinal chip as well as a specialised pair of AR eyeglasses, whereby each photovoltaic pixel (on the implant) is independently controlled and self-powered by near-infrared light projected from glasses worn by the patient (the glasses consist of a camera and digital mirror projector, which emit a near infrared light pattern through the patient’s eye carrying the Prima implant, designed to be processed by the Prima implant’s pixels/electrodes). As part of these upgrades, Pixium will be implementing a new version of the AR glasses (Prima 2), which we expect to incorporate more advanced imaged processing and artificial intelligence functionality to enhance the visual experience of patients implanted with the current 378-electrode Prima chip.
To this end, the firm expects to pursue additional European feasibility testing on the second-generation upgraded system (employing Prima 2 glasses) in the five patients already enrolled and implanted in the existing EU feasibility study. We also expect patients to be implanted in the ongoing US feasibility study will be employing this upgraded system. Data from both EU and US feasibility trials will be used in the planning and development of the EU pivotal study.
We expect the current iteration of the Prima implant chip (378-electrode count) to remain unchanged until the firm receives an initial market approval. Once the Prima system receives market approval or clearance, we expect the company will then investigate human trials using higher-density Prima chips, which can theoretically provide higher visual resolution when implanted in patients. A discussion on next-generation Prima implant chips was included in our Outlook report.
EU pivotal study to now start in H120
Prior to commencing a CE-mark enabling EU pivotal study, Pixium prefers to have results on hand from feasibility studies with the upgraded Prima system (ie whether the new analytics and other improvements from Prima 2 will improve visual functionality and quality of life parameters). The company believes that having such data would raise the likelihood of success in the pivotal program, and could also better help coordinate development approaches for Prima in both the EU and US markets. Given its new strategy to obtaining human feasibility data on the upgraded Prima system first, the company has pushed back its expected timeline for commencing an EU pivotal study to H120, from H219, previously. However, Pixium is also pursuing several regulatory and clinical approaches to potentially accelerate development (and eventual product launch) and it has ongoing discussions with the EU and US regulatory authorities. Nonetheless, we are pushing back our base-case Prima commercialisation timeline assumptions.
We continue to estimate the EU pivotal study may require 40–50 patients and will require 12 months of follow-up safety and efficacy data for European regulators to provide CE mark approval. We now estimate that 12-month data from the EU pivotal study will be available in H122 (from H221, previously), leading to potential EU commercialisation (CE mark approval) in 2023 (from H222, previously). We expect that CE mark clearance (and EU launch) would occur 18–24 months earlier than US pre-market approval (PMA) and launch. We now model a potential US launch in 2025 (from 2024, previously).
Prima financial assumptions and forecasts
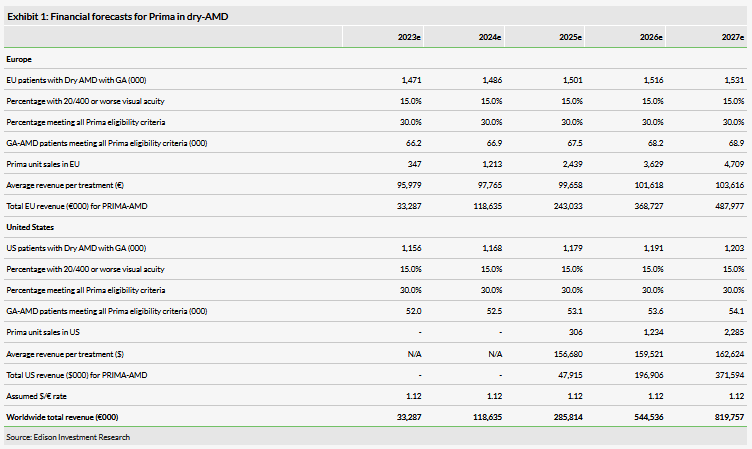
As stated in our Outlook report, we estimate that the prevalence of geographic atrophy (GA) associated with dry-AMD would currently be approximately 1.1 million in the US and approximately 1.4 million in Europe.
Given that the current Prima iteration appears to provide VA in the 20/460 to 20/550 range (based on feasibility data to date), we continue to estimate that the target population will be those GA patients with below 20/400 (5%) VA, and we believe that this would represent about 15% of GA patients. In other words, we estimate 15% of patients with GA would have sufficiently poor central vision to warrant potential consideration for Prima. Of these, we estimate that 30% would meet all remaining inclusion criteria and/or be suitable as potential responders (ie this considers that many of the AMD patients are in poor general health and/or have concomitant eye diseases, such as glaucoma or poor optical media transparency, which would render them ineligible for Prima). Given the above, we now estimate the target eligible GA-AMD treatment population for the current 378-electrode Prima to be currently about 63,000 in Europe and 49,500 in the US. Our peak market share forecasts (of the eligible treatment population) remain unchanged at 7%. As we have pushed back our launch timelines as described above, our new financial forecasts are shown below.
Financials
We believe that Pixium’s mid-2019 funds on hand (€10.2m) should be sufficient for the company to maintain its operations and fund its Prima strategy into Q220. As stated previously, we calculate €1.9m in net cash after adjusting for €8.35m of gross debt (€2.49m in refundable advances, €4.38m venture loan, and €1.48m in short- and long-term lease debt).
We have pushed back portions of our prior H219 and beyond R&D expenditure forecasts by about six months, given that the EU pivotal study is now only expected to start in H120 (vs prior assumptions of Q419). Altogether, we expect EU pivotal study related costs to peak in 2021 (as recruitment reaches completion). We now forecast 2019 and 2020 operating cash burn rates (excluding net interest) of €10.4m and €15.8m, respectively, versus our prior estimates of €9.7m and €16.9m, respectively.
We expect that Pixium will seek to raise funds in H219, in order to expand its financial runway to fund the EU pivotal study. Our model continues to estimate that Pixium will raise €20m in H219 and €30m in 2020 in funding. We have increased our 2021 funding projection to €33m, from €25m previously, given that commercialisation and sales ramp for Prima will now occur a bit later than previously expected. As per usual Ed ison policy, our model assumes these sources will be in debt. We forecast th at all this funding should enable Pixium to complete the registration-enabling Prima clinical studies in the EU to reach commercialisation in Europe. In addition, positive cash flows resulting from EU sales should enable the completion of the US pivotal study. We continue to assume that Pixium will only start to become cash flow positive on a sustainable basis once Prima is launched (in 2023).