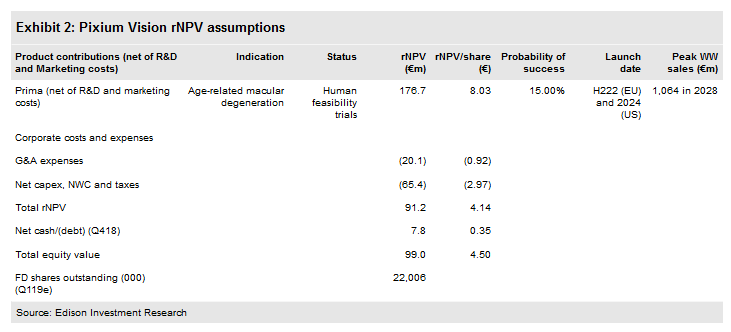
Pixium Vision SA (PA:PIX) reported FY18 financials and reiterated that it is on track to start a pivotal study in the EU for its Prima bionic vision system (BVS) in H219 for the treatment of advanced dry age-related macular degeneration (Dry-ARMD). This follows the release of positive six-month data in January 2019 for its five-patient EU Prima feasibility study. Using a risk-adjusted NPV model, we obtain a pipeline rNPV of €91.2m, vs €88.7m previously.
FY18 financials reveal no negative surprises
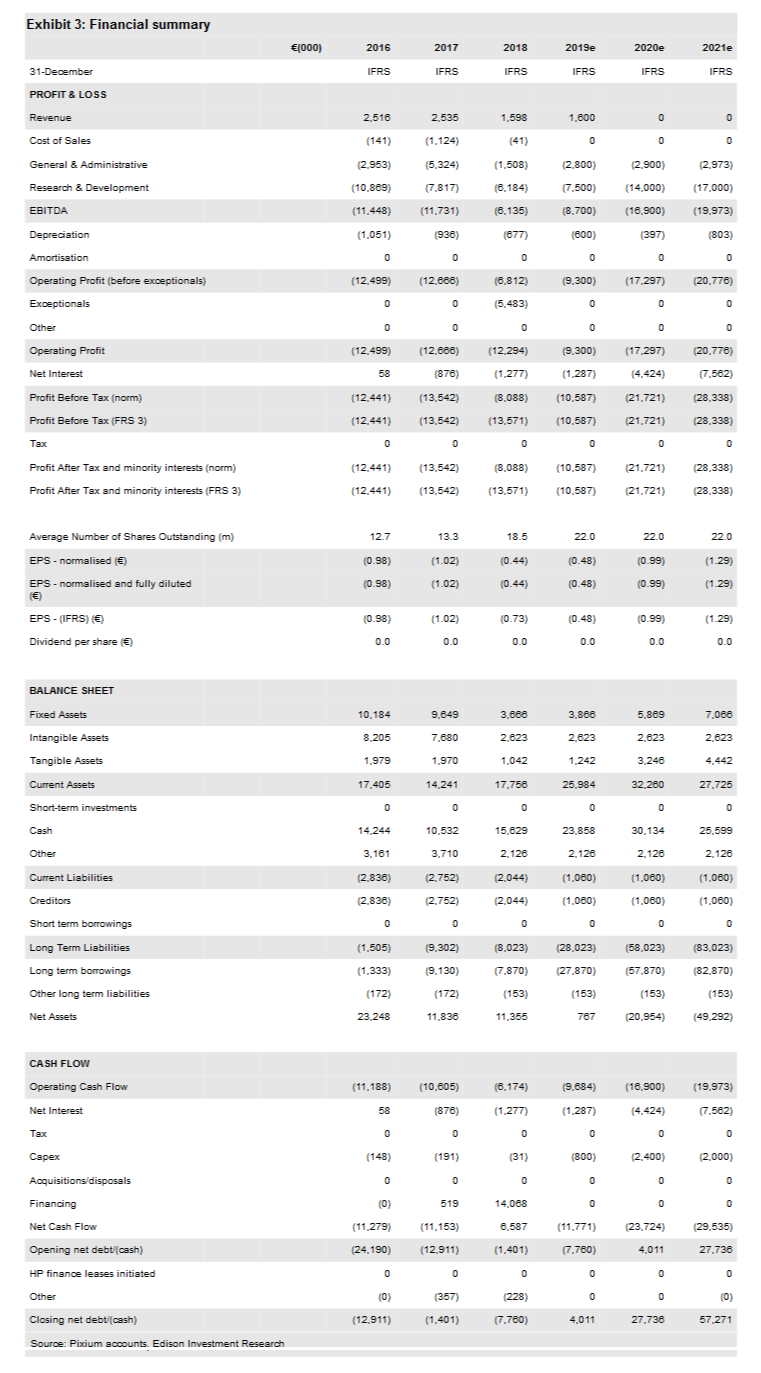
Pixium reported Q418 gross cash and equivalents of €15.6m, and a yearly operating cash burn rate of €6.17m excluding net interest/finance costs of €1.28m. The sharp reduction in the operating loss from €12.67m in 2017 to €6.81m was, as expected, due to the cessation of the Iris II programme, which resulted in significantly lower COGS and marketing expenses, as well as slightly lower R&D costs.
EU pivotal study expected to start in H219
Pixium plans to work with study investigators and statisticians in coming weeks to analyse full study data and formulate a pivotal study design. It will then begin discussions with regulators to conduct a pan-European pivotal trial across several countries and multiple centres. Pixium’s goal is to start recruitment for the pivotal study in H219, potentially resulting in initial implantations before YE19. We estimate it will require 12 months of follow-up data within the EU pivotal trial for European regulators to provide CE Mark approval. We believe that the EU pivotal study may require 40–50 patients and that EU commercialisation (CE Mark approval) may occur in H222.
Valuation: €99.0m in equity, or €4.50 per share
We believe Pixium’s cash on hand should be sufficient for it to maintain its operations into Q220. Our model continues to estimate that Pixium will raise €20m in 2019, €30m in 2020 and €25m in 2021. As per Edison policy, we model these as debt financing. We continue to value Pixium using an rNPV approach, employing a 12.5% cost of capital and applying a 15% probability of success estimate for Prima. After rolling forward our estimates and reducing near-term R&D costs, we now obtain a pipeline rNPV (enterprise value) of €91.2m, up from €88.7m, previously. After including €7.8m in Q418 net cash (inclusive of €7.9m gross debt) at 31 December 2018, we obtain an equity valuation of c €99.0m, or €4.50 per share (compared to €4.42 previously).
Business description
Pixium Vision develops bionic vision systems for patients with severe vision loss. Its lead product, Prima, is a wireless sub-retinal implant system designed for Dry-ARMD. The company has completed a human feasibility study for Prima in Europe and expects to start implantations in a US feasibility study in H119.
Prima strategy on track with expectations
Pixium Vision provided a general business and financial update on 8 February 2019. The company is focused on advancing its Prima bionic vision system for the treatment of Dry-ARMD. The Prima implant is a miniaturised photovoltaic wireless sub-retinal microchip consisting of 378 electrodes (pixels) that is placed underneath the retina. Each photovoltaic pixel is independently controlled and self-powered by near-infrared light projected from the specialised glasses worn by the patient.
On 8 January 2019, Pixium announced that Prima successfully met the endpoints of the five-patient EU feasibility study1 at interim six months follow-up after implantation, in patients with Dry-ARMD. Pixium indicated that these results exceeded its initial expectations, as all five implantations resulted in successful activations and light perception in areas where no central vision remained prior to implantation. Most patients were able to identify different visual patterns, symbols or letter sequences, and recognition speed improved throughout the post-implantation rehabilitation phase. Some patients reported visual acuity (VA) measures of up to 20/460, which to our knowledge is among the highest level recorded with a prosthetic retinal implant device. Safety measures to date suggest the implant is stable and well-tolerated, as there were no device-related serious adverse events and the device does not impair residual natural peripheral vision.
In parallel, the company continues to advance with the single-centre, five-patient US feasibility trial (PRIMA FS-US). Conducted at the University of Pittsburgh Medical Center, it is actively recruiting and screening potentially eligible patients. Management expects the first implantations to occur in H119. The public release of interim data from the European feasibility study could encourage patient recruitment for this US study. Pixium believes that 12-month safety and performance data on all five patients will likely be sufficient for US regulators to allow a larger US (pilot) study to be started. We continue to anticipate that study data from the US feasibility study should be available in H120 and that recruitment for the US pilot study can begin in H220.
Pixium now focused on gaining approval for EU pivotal study
As reiterated in our 17 December 2018 note, the regulatory pathway for a European approval is shorter than in the US and Pixium is confident the interim safety data from the European feasibility study can be used to enable the design of the protocol for a larger, multi-centre, CE Mark-enabling European pivotal study. The company plans to work with study investigators and statisticians in coming weeks to analyse full study data and formulate a pivotal study design. It will then begin discussions with regulators to conduct a pan-European pivotal trial across several countries and multiple centres. Pixium’s goal is to start recruitment for the pivotal study in H219, potentially resulting in initial implantations before YE19. The firm expects the EU pivotal study to involve sites in several countries, including France, Germany, Italy and Spain.
We continue to estimate it will require 12 months of follow-up safety and efficacy data within the EU pivotal trial for European regulators to provide CE Mark approval. We estimate that the EU pivotal study may require 40–50 patients. We reiterate that to obtain CE Mark approval product safety is generally the primary consideration for regulators (it does not usually require demonstration of long-term clinical efficacy). We continue to estimate that 12-month data from the EU pivotal study (which we estimate is the minimum required for approval) will be available in H221, leading to potential EU commercialisation (CE Mark approval) in H222. We expect that CE Mark clearance (and EU launch) would still occur 18–24 months earlier than US pre-market approval (PMA) and launch.

Review of FY18 financial results
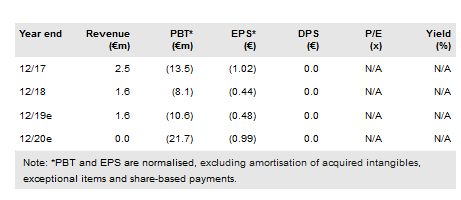
Pixium reported Q418 gross cash and equivalents of €15.6m and a 2018 operating cash burn rate of €6.17m excluding net interest/finance costs of €1.28m. The firm reported €1.60m in revenue in FY18 (primarily from subsidies and research tax credits), down from €2.54m in FY17. It realised a €6.81m operating loss2 (vs a €12.67m loss in FY17), and a €13.57m net loss (€0.73 per share), vs a €13.54m net loss in FY17.
Included within the FY18 net loss was a one-time €5.48m impairment charge related to tangible and non-tangible assets relating to the now-discontinued earlier-generation Iris II epi-retinal implant programme. Excluding this impairment charge, the company’s adjusted net loss was €8.09m, or €0.44 per share.
Overall, Pixium’s sharply lower operating and adjusted net losses compared to FY17 were, as expected, due to the cessation of the Iris II programme, which resulted in significantly lower COGS and marketing expenses, as well as slightly lower R&D costs.
Pixium’s results compare to our FY18 expectations of operating and normalised net losses of €6.7m and €6.49m respectively. The normalised net loss was higher than we expected primarily due to higher than expected net finance costs (€1.28m). Reported FY18 R&D costs (€6.18m) were only slightly ($0.14m) higher than we projected.
Financial outlook
We expect Pixium’s R&D costs and rate of cash burn to increase in 2019 as:
it prepares for the EU pivotal study
the US feasibility study progresses
it finalises processing and design enhancements to the Prima system’s external glasses, and
it produces Prima microchip and glasses inventory for the EU pivotal study.
We believe that Pixium’s funds on hand (€15.6m) should be sufficient for the company to maintain its operations and fund its Prima strategy into Q220. Given that the firm reported €7.9m in total gross debt on 31 December 2018 (€2.4m in conditional advances and €5.5m in long-term debt), we calculate €7.8m in net cash.
Following discussions with management, we have slightly reduced our R&D cost assumptions for the EU pivotal study, particularly in 2019 where we estimate that implantation numbers will be very limited. We anticipate EU pivotal study patient recruitment and implantations to increase significantly in 2020, and that implantations will then also begin for the US pilot study, resulting in a yearly increase in R&D costs.
Compared to our prior estimates, we have lowered our R&D expense assumptions in 2019 and 2020, to €7.5m and €14.0m, respectively (from €10.5m and €16.0m, respectively). We now forecast 2019 and 2020 operating cash burn rates (excluding net interest) of €9.7m and €16.9m respectively, vs our prior estimates of €12.5m and €21.0m, respectively.
We anticipate Pixium will seek to raise funds, likely in mid-2019 or H219, in order to expand its financial runway to fund the EU pivotal study. Our model continues to estimate that Pixium will raise €20m in 2019, €30m in 2020 and €25m in 2021. As per usual Edison policy, our model assumes these sources will be in debt. We forecast that all this funding should enable Pixium to complete the registration-enabling Prima clinical studies in the EU to reach commercialisation in Europe. In addition, positive cash flows resulting from EU sales should enable the completion of the US pivotal study. We continue to assume that Pixium will only start to become cash flow positive on a sustainable basis once Prima is launched (in H222).
Valuation
We continue to value Pixium using an rNPV approach, employing a 12.5% cost of capital. Our valuation is based solely on the Prima opportunity in Dry-ARMD. We continue to apply a probability of success estimate for Prima-ARMD in our model of 15% and we assume a forex rate, for US sales, of $1.13/€.
We have not revised our Prima market share, revenue or peak sales assumptions, although we have rolled forward our forecasts in our valuation model and reduced our 2019 and 2020 R&D expense forecasts as stated previously. Hence, we now obtain a pipeline rNPV (enterprise value) of €91.2m, up from €88.7m previously. After including €7.8m in net cash at 31 December 2018, we obtain an equity valuation of c €99.0m, or €4.50 per share (compared to €4.42 previously).