Oxford BioMedica PLC (LON:OXB)’s interim results highlight strong operational and financial momentum to date. The Novo Holdings equity investment (£53.5m) in May has enabled OXB to fully repay the debt facility, effectively strengthening the balance sheet. It is investing ahead of increasing demand for its lentiviral vector manufacturing capacity with the build-out of OxBox. The new facility will more than double capacity and is expected to be ready for commercial vector production in H120. Top-line growth continues to benefit from the near-term ramp-up of Kymriah and rapid advancement of partnered asset AXO-Lenti-PD (Axovant), crystallising in a $15m development milestone payment. We value OXB at £673m.
Partnerships advancing through financial milestones
Partner Axovant has swiftly accelerated AXO-Lenti-PD into the clinic, with a Phase I/II dose-escalation study (SUNRISE-PD) in advanced PD patients ongoing. Following positive early data in the lowest dose cohort (n=2), a second dose cohort is enrolling (OXB received a $15m milestone payment on enrolment of first patient) for which data are expected by year end. We anticipate further milestone payments (up to $40m remaining in development milestones) as progress continues. The haemophilia development partnership with Sanofi (PA:SASY) (previously Bioverativ) is progressing towards clinical development material in the coming 12 months, which could begin to trigger milestones (up to $100m). With Kymriah now approved for reimbursement in over 19 countries, we expect OXB to benefit from an increasing royalty stream. OXB continues to garner new partners including R&D collaborations with Santen (gene therapy for an inherited retinal disorder) and Microsoft (NASDAQ:MSFT) (to improve the yield and quality of vectors utilising machine learning).
Debt removed, investment is the strategy
Novo’s £53.5m investment has multiple consequences. It has enabled OXB to fully repay the costly revolving debt facility, leading to a much improved debt-free balance sheet, and boosted cash for developing its platform and own portfolio of assets. Capital expenditure increased to £14.9m in H119, highlighting the significant investment in OxBox (84,000 sq ft bioprocessing manufacturing facility), which is a pre-requisite of meeting future demand. In addition, a 32,000 sq ft discovery facility has been leased to boost R&D capabilities.
Valuation: £673m vs £649m previously
We value OXB at £673m (£8.77/share) vs £649m previously, as a result of updating for FY19 cost and revenue forecasts, rolling forward our model and updating for exchange rates and net cash. Our sales forecasts are unchanged and our core drivers remain OXB’s partnerships, which represent £5.36/share of our total value.
Business description
Oxford Biomedica’s (OXB) LentiVector technology underpins the company’s strategy. OXB generates significant revenue from partners that use its technology, notably Novartis, Bioverativ (Sanofi (PA:SASY)), Orchard Therapeutics and Immune Design. OXB is implementing significant capacity upgrades to enable more partnering/out-licensing agreements.
Balance sheet strengthened as partners progress
We continue to expect ongoing growth in the top line, driven in the near term by Kymriah (Novartis), the progression of Sanofi’s (previously Bioverativ) haemophilia products to the clinic (via development milestone payments) and the rapid advancement of OXB’s partnered products with Orchard (notably OTL-101, where a biologics licence application (BLA)/marketing authorisation application(MAA) is expected in 2020) and Axovant (cohort 2 data expected in Q419). Additionally, early-stage collaborations with Santen and Boehringer Ingelheim/the UK Cystic Fibrosis Gene Therapy Consortium will continue to contribute a growing share of the top line as OXB undertakes development work.
The equity investment in May of £53.5m by Novo Holdings (for 10.1% of the outstanding share capital) has enabled OXB to fully repay the £43.6m debt facility with Oaktree Capital Management. This is a welcome move as the debt cost was substantial (as evidenced by the H119 interest payment of £4.8m) and its removal will allow OXB to fully focus its cash resources on growing the business.
To this end, OXB has made significant progress in H119 in building out its manufacturing and R&D capacity in addition to its workforce. In September 2018, the group signed a lease on 84,000 sq ft facility located about a mile from the main residence. Initially the company will fit out approximately 45,000 sqft with four GMP clean room suites, two fill and finish suites, offices, warehousing and QC laboratories. Completion of the building phase is expected by the end of 2019, with validation of the manufacturing and necessary regulatory approvals expected by the end of the second quarter 2020. In December 2018, OXB signed a lease on a 32,000 sq ft building adjacent to its main residence in Windrush Court. The new facility, named the Windrush Innovation Centre, will focus on innovation and technological advances to support both the product pipeline and LentiVector platform. Teams started moving into the facility in Q219 and the centre is expected to be fully operational within a year. At 30 June 2019, the employee count had risen to 465 from 352 at 30 June 2018 and the company expects it to rise to 600 staff by year end. Hiring the correct talent will be critical to OXB’s long-term success.
OXB proving its worth as Kymriah fights headwinds
OXB’s successful transition to Process B bioreactor vector production for Kymriah (a tenfold yield over Process A) in H119 demonstrates its expertise in vector manufacturing. Due to low Kymriah sales to date (Q219: $58m), vector supply has been possible with adherent cell manufacturing (Process A). However, to make sure Kymriah sales in the long term are not limited by vector supply, a transition to bioreactors was needed.
In July 2017, OXB and Novartis signed a three-year commercial supply agreement in support of Kymriah that comes to an end in July 2020. We believe OXB and Novartis are currently likely to be in discussions to extend this contract. OXB’s technology is deeply embedded in Kymriah’s production and swapping to another vector manufacturer (either in house at Novartis or external) would require a significant technology transfer undertaking (12–18 months of work), which would materially affect Novartis’s ability to supply Kymriah into H220 and beyond.
Kymriah is now approved for reimbursement in 19 countries. However, it continues to face headwinds that have slowed uptake. In August, the Centers for Medicare and Medicaid Services (CMS) announced that current CAR-T treatments (Kymriah and Yescarta) will now be reimbursed by Medicare when patients are treated at approved healthcare centres. However, the amount reimbursed remains contentious, with many hospitals claiming these new rules do not cover the total cost of treatment.
While OXB provides the vectors that encode a patient’s T-cells to express the cancer-targeting chimeric antigen receptor (CAR), it is Novartis that undertakes the cell engineering of a patient’s cells. This is a complex and difficult process and, since the launch of Kymriah, Novartis’s cell manufacturing has come under scrutiny as some Kymriah products have not met commercial specification requirements (although they did meet clinical requirements). As a result, some Kymriah products had to be provided to patients for free; while this affects royalties received by OXB, the company still receives vector batch payments. A request to broaden the specification requirements has been approved by the EMA, although the FDA has yet to alter its requirements.
In addition to Kymriah, Novartis and OXB are working on a second undisclosed CAR-T, which is expected to progress into the clinic in the next 12 months.
AXO-Lenti-PD hitting development milestones
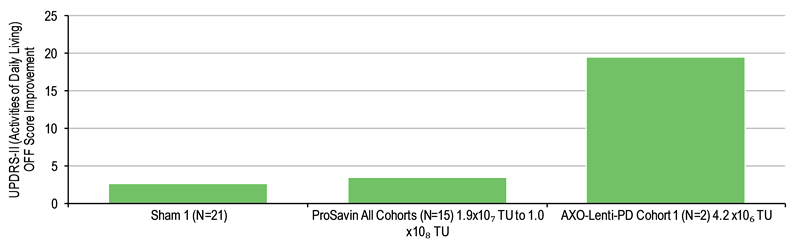
Partner Axovant has swiftly undertaken clinical development of AXO-Lenti-PD, with three-month data published early this year from the first two patients in the ongoing Phase I/II dose-escalation study (SUNRISE-PD) in advanced PD patients. Updated six-month data were announced in June. The study highlighted mean improvements (Exhibits 1 and 2) in Unified Parkinson’s Disease Rating Scale (UPDRS) scores for motor function (Part III) of 17 points (individual patients demonstrated 14- and 20-point improvements) and daily living (Part II) of 20 points at the lowest dose to be tested of 4.2 x 106 transducing units (TU). This compared favourably to that observed for the highest dose used in the ProSavin study (Exhibits 1 and 2). The next data are expected in Q4 from the second cohort of patients treated at 1.4 x 107 TU. OXB is eligible for remaining milestones of up to c $800m ($40m left for development) and 7–10% tiered royalties on any sales. The Axovant partnership currently represents 23.4% (£2.07/share) of our value of OXB.
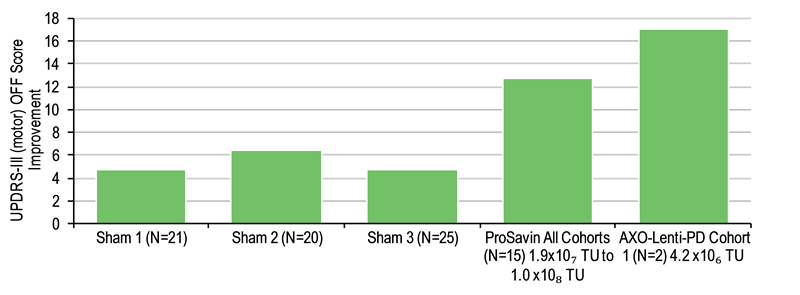
Exhibit 1: UPDRS-III score improvement after six months for motor function
Exhibit 2: UPDRS-II score improvement after six months for daily living
New collaborations add to the mix
In June, OXB signed an R&D collaboration, in addition to an option and licence agreement with Santen Pharmaceuticals, a publicly listed, leading Japanese ophthalmic pharmaceutical company operating in 60 countries worldwide. The agreement covers the development of gene therapy vectors for an undisclosed rare inherited retinal disorder. Under the terms of the initial agreement, OXB will provide preclinical proof of concept with its lentiviral vector platform. The collaboration includes a licence to OXB’s LentiVector platform and access to its manufacturing facilities. OXB is entitled to an undisclosed milestone payment on Santen exercising the option to the LentiVector platform, as well as development milestones and an up to 10% royalty on net sales.
Santen has worldwide commercial rights to the programme, while OXB retains an option to co-fund and participate in the development and commercialisation in the US and Europe. The structure of this deal is interesting as it is of minimal risk at this early stage , but provides the opportunity to share in the upside if this gene therapy has commercial viability in the US/EU.
Additionally, in an effort to ensure it remains at the cutting edge, in March OXB announced a collaboration with Microsoft (NASDAQ:MSFT) Research to utilise its machine learning technology to develop insights into OXB’s processes. The aim of the partnership is to improve the yield and quality of OXB’s next-generation gene therapy vectors. The partnership will initially run for two years, but can be extended by either party.
Financials
OXB reported H119 gross income of £32.1m (-9% y-o-y from £35.3m). Licence fees, milestone and royalty (LMR) revenue dropped to £13.3m in H119, as H118 (£19.9m) benefited from large upfront payments from signing the Axovant and Sanofi (PA:SASY) (Bioverativ) partnerships. LMR revenue in H119 comprised an £11.5m ($15m) milestone from Axovant (actual cash received post period) and, although undisclosed, we assume the remainder is £1.8m in royalties for Kymriah.
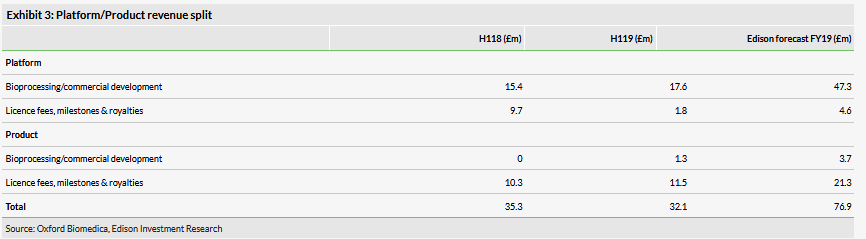
Bioprocessing/commercial development revenues (segmental data in Exhibit 3), which are historically more predictable, grew to £18.8m (+23% y-o-y from £15.4m), driven by a greater volume of development activity, notably for Novartis and Orchard. Production of clinical and commercial batches for Novartis and Orchard were slightly lower y-o-y, as production was affected by conversion of the Yarnton bioprocessing facility from an adherent process (Process A) to bioreactors (Process B). Additionally, OXB has now been approved by the US FDA to provide vectors produced by cell Process B instead of the original (at time of filling) Process A vectors. This has the additional effect of lowering the required number of vector batches OXB needs to provide to Novartis as Process B can provide 10x the vector quantity per batch than Process A (enabling 10x the amount of royalties paid to OXB per batch). However, as the number of patients treated with Kymriah continues to grow, we expect bioprocessing revenues to pick up once again.
We have made minor adjustments to our FY19 revenue forecasts of £76.9m (from £75.8m previously). R&D and COGS increased to £17.6m in H119 (H118: £13.4m) and £11.7m (H118: £10.1m) respectively. For R&D, this was a result of increased investments in commercial and technical projects, while the increase in COGS was driven by growth in bioprocessing. R&D has grown more quickly than we originally forecast, and we have therefore adjusted our FY19 R&D forecasts upwards to £44.3m (vs £31.9m previously). Our FY19 COGS forecast remains broadly in line with our previous assumptions (£24.2m vs £24.0m previously). Admin costs rose to £4.0m (vs £2.4m in H118) and we retain our full year forecast of £10.0m.
We note that bioprocessing costs increased to £4.1m (vs £0.7m in H118) as a result of headcount, facility costs and related spend on OxBox. Costs were also affected by the downtime at the Yarnton bioprocessing facility (switch from Process A to B) where the associated downtime costs were accounted for in bioprocessing costs rather than as COGS (as no goods were sold in the downtime). We currently include forecast bioprocessing costs in our R&D line.
Finance costs of £6.1m (H118: £4.2m) consisted of interest payments on OXB’s loan facility with Oaktree Capital Management, which increased to £4.8m (H118: £3.0m), a foreign exchange revaluation loss of £1.0m driven by weak sterling (vs a loss of £1.2m in H118) and lease liability interest (as recognised under IFRS 16) of £0.3m. In the period, OXB repaid the Oaktree loan (interest rate of 9% plus Libor, subject to a minimum of 1%) following Novo’s equity investment of £53.5m. Tax credits of £1.9m were received in H119 versus none in H118.
Capital expenditure in H119 was £14.9m (vs £6.0m in H118), driven mainly by the ongoing build and fit of OxBox and the expansion of the business as a whole. Capex was substantially above expectations and we now forecast FY19 capex of £26.3m (vs £14.1m previously).
Gross and net cash was £26.1m at 30 June (vs gross cash of £32.3m and net debt of £8.9m at 31 December 2018). This does not include the £11.5m Axovant upfront, which is expected in H219.
We now forecast a £6.6m net loss in FY19, but note that multiple sensitivities remain around this figure, including cost sensitivities in R&D, facilities and personnel, in addition to revenue sensitivities with regard to Kymriah sales growth, the extent of bioprocessing revenue, milestone payments and the execution of any new deals.
Valuation: £673m (£8.77/share)
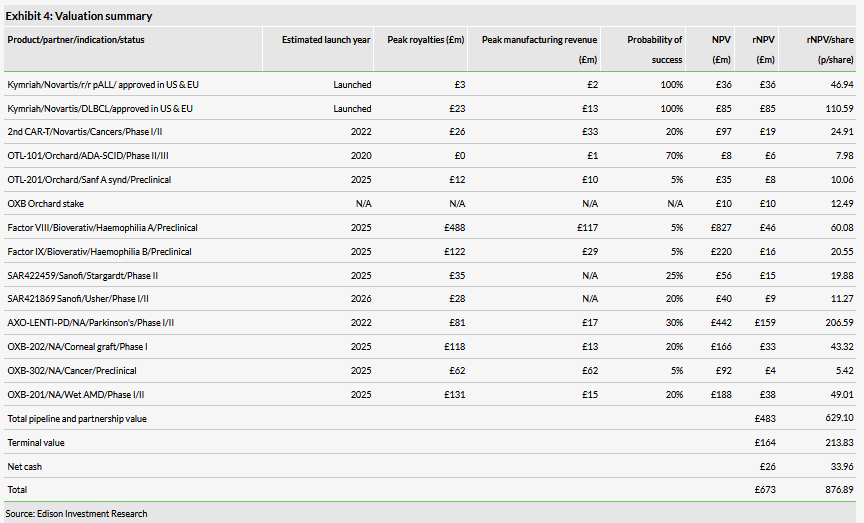
We value OXB at £673m (£8.77/share) vs £649m previously, as a result of updating for FY19 cost and revenue forecasts, rolling forward our model, and updating for exchange rates and net cash. Our valuation is based on a risk-adjusted NPV of partnered products with Novartis (Kymriah and undisclosed second CAR-T: £1.82/share), Orchard Therapeutics (OTL-101 and OTL-201: 18p/share + 12p/share for the equity stake), Bioverativ/Sanofi (Factor VIII and Factor IX: 81p/share), Sanofi (PA:SASY) (SAR422459 and SAR421869: 31p/share), AXO-Lenti-PD (PD: £2.07/share), OXB-201 (wet AMD: 49p/share), OXB-202 (corneal graft rejection: 43p/share) and OXB-302 (cancer: 5p/share). We include net cash (33p/share) and a terminal value (£2.14/share).
For extensive details of our valuation, please see our outlook note, In a cell and gene therapy sweet spot.