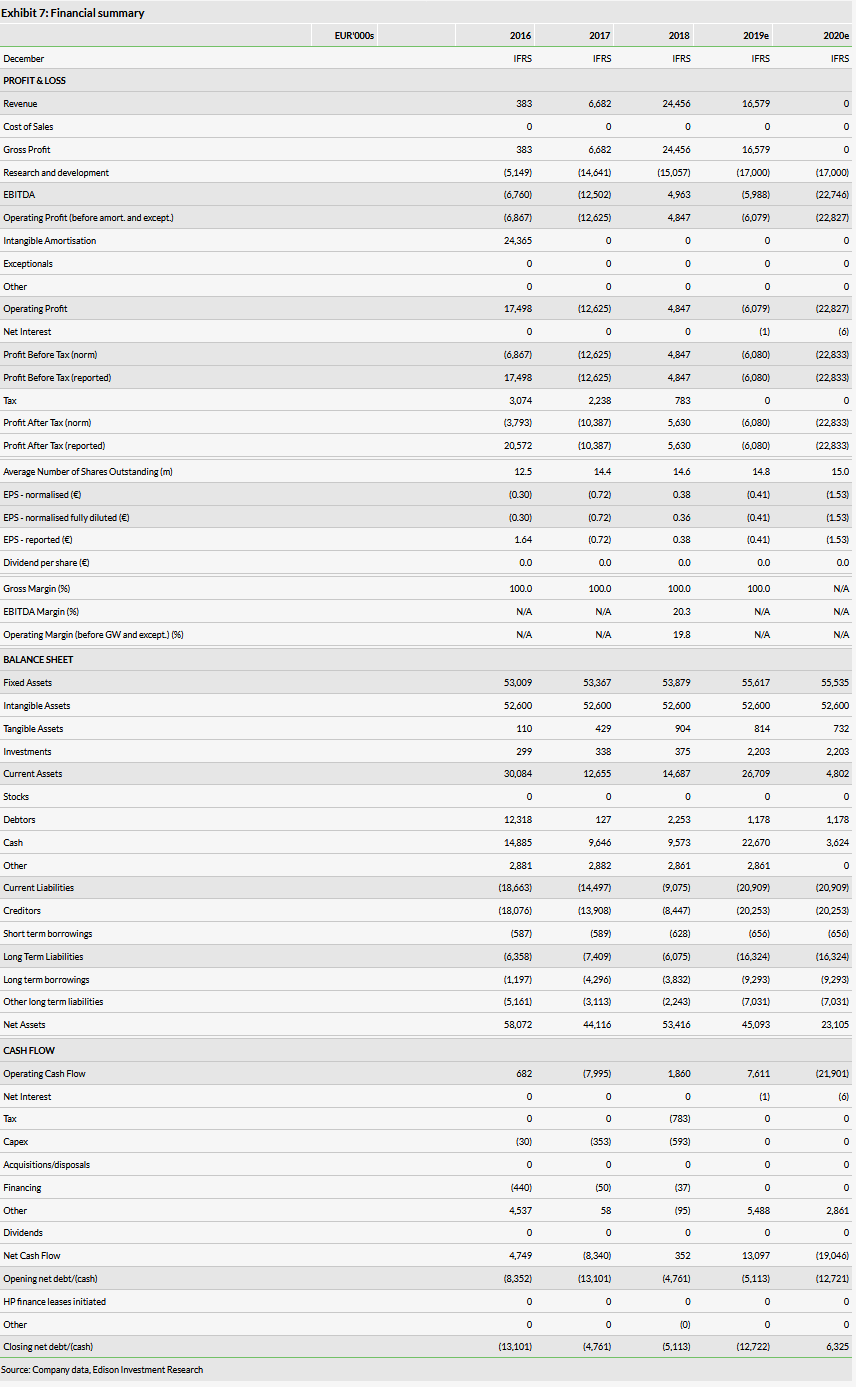
OSE Immunotherapeutics (PA:OSE) now has four clinical trials running. Interim results from its Phase III Atalante 1 trial with cancer vaccine Tedopi are a key catalyst and due in Q120. Another three trials were initiated over the last 12 months, which brought OSE a total of €27m in payments from partners (plus another €5.4m from Bpifrance). These trials include a Phase I study with OSE-127 (partnered with Servier), a Phase I study with BI 765063 (previously OSE-172; with Boehringer Ingelheim, BI) and a Phase II study with Tedopi in pancreatic cancer (GERCOR). We raise our valuation of OSE to €198m or €13.2/share (previously €190m).
Phase III Atalante 1 interim results in Q120
Although OSE has a broad and diversified R&D pipeline, much of the focus until early 2020 (Q120) will be on the Phase III Atalante 1 trial with a peptide cancer vaccine Tedopi for second- and third-line treatment of non-small cell lung cancer patients (NSCLC). OSE hosted a Tedopi-focused KOL event in New York in May 2019, where the presenters provided a detailed overview of the cancer vaccine’s development and of target indications NSCLC and pancreatic cancer.
Self-sufficient R&D
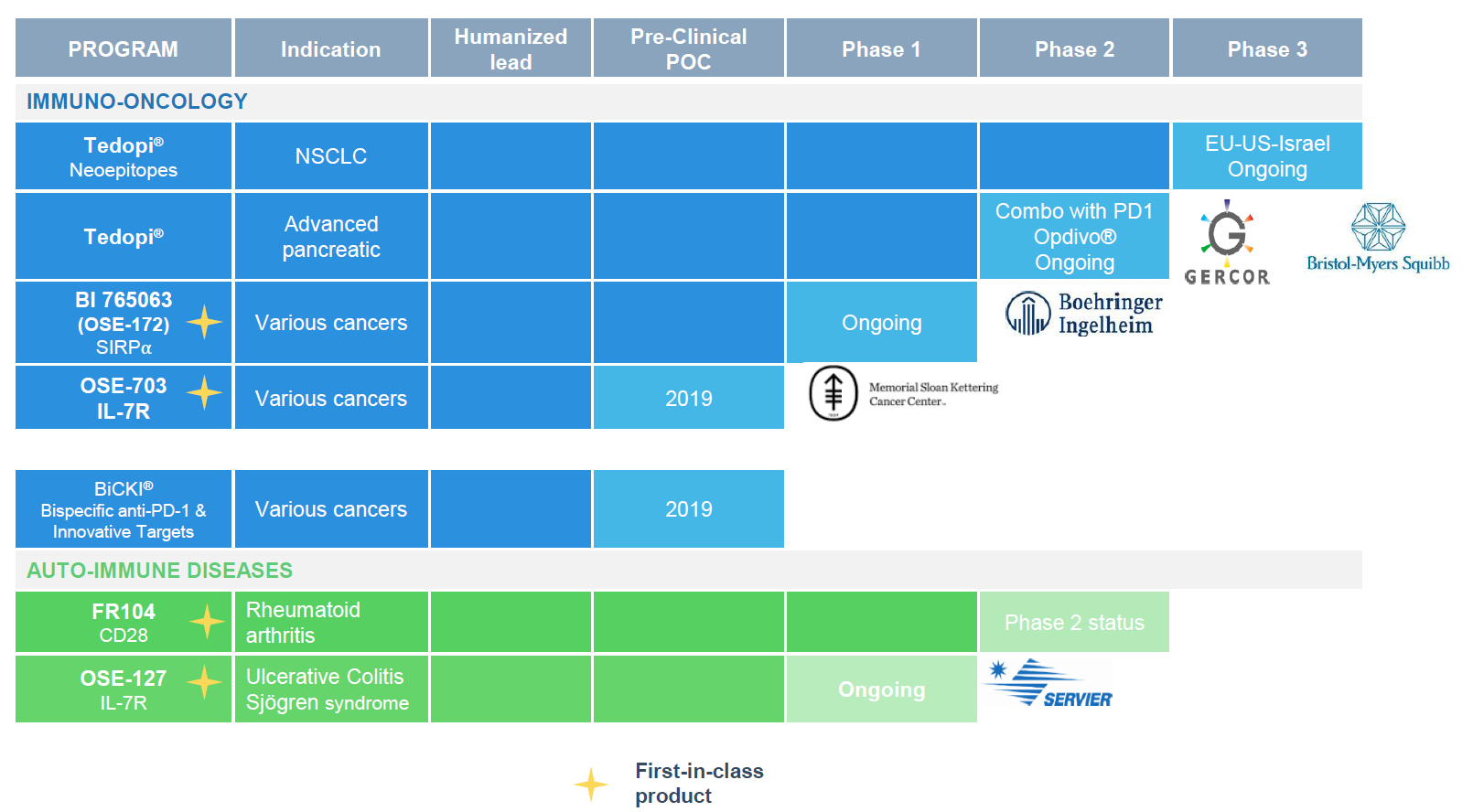
In addition to Phase III Atalante 1 (funded by OSE), Tedopi is being tested in a Phase II trial in pancreatic cancer patients, which is run by OSE’s partner GERCOR (a physician network). Another two trials were initiated earlier this year and both are financed by partners. These include a Phase I study with BI 765063 (OSE-172), a SIRPα antagonist for solid tumours, which is run by OSE but funded by BI, which paid €15m in milestones on initiation of the trial. According to the terms of the licensing deal with BI, OSE is still eligible to receive up to €1.1bn in milestones plus royalties if certain conditions are met. Similarly, OSE initiated a Phase I trial with its OSE-127 IL-7Rα (CD127) antagonist for autoimmune diseases, which triggered a €12m payment (Servier exercised an option; total value of the deal €272m, of which €22m has been received).
Valuation: €198m or €13.2/share
We have raised our valuation of OSE to €198m or €13.2/share from €190m or €12.8/share previously, which is due to a better cash position, rolling our model forward and increasing the probability of success to 10% for BI 765063 (OSE-172) as the Phase I trial has been initiated. The milestone payments from Servier and BI had virtually no effect as we have already included them in our model. The interim results from the Phase III Atalante 1 trials are the main catalyst in the near term.
Business description
OSE Immunotherapeutics is an immunotherapy company based in Nantes and Paris, France, and listed on the Euronext Paris exchange. OSE is developing immunotherapies for the treatment of solid tumours and autoimmune diseases and has established several partnerships with large pharma companies.
Tedopi KOL event; interim Atalante 1 trial data in Q120
On 30 May 2019, OSE hosted a KOL day in New York, which focused on Tedopi, NSCLC and pancreatic cancer. The presentations provided more detailed background on the development of Tedopi and an overview of the target cancer indications. Overall, we view the update as in line with our discussions in our initiation report. Although OSE has a well-balanced R&D pipeline in terms of asset stages and technology types, the focus on Tedopi will prevail due to a combination of factors. It is the most advanced OSE asset in development and interim analysis will be carried out in Q120, which will also include some efficacy data; this makes it a substantial catalyst for OSE.
Atalante 1 is a Phase III trial with Tedopi as second- or third-line treatment against standard of care (docetaxel or pemetrexed) in HLA-A2 positive patients with locally advanced (IIIB), unsuitable for radiotherapy or metastatic (IV) NSCLC. Post-checkpoint failure patients represent an area where no novel treatment has been approved yet. The trial aims to enrol 300 patients. Interim analysis is expected in Q120, which will include survival data from the first 84 patients. This is a significant catalyst for OSE, although the final results should come later in 2021. OSE presented case reports from three patients from the Atalante 1 trial at AACR in Atlanta on 31 March 2019, which we discussed in our last update report.
At the end of 2018, OSE’s partner GERCOR (a physician network) initiated a Phase II trial with Tedopi as maintenance therapy alone or in combination with Opdivo or FOLFIRI in pancreatic cancer after induction therapy with FOLFIRINOX. Bristol-Myers Squibb (NYSE:BMY) is supporting the trial by providing Opdivo for free. Results are expected in 2022. Pancreatic cancer represents an unmet need with relatively little advance in treatment compared to most other cancers over the last decade. Pancreatic cancer is a ‘cold’ tumour, so the rationale is to combine immune-priming cancer vaccine Tedopi (stimulates specific T-cell response) with checkpoint inhibitors (makes tumours ‘visible’ to the immune system). OSE will evaluate options for further development and commercialisation depending on the results of this study.
Exhibit 1: OSE’s pipeline
BI 765063: First patient enrolled in Ph I, milestone paid
OSE announced on 17 June 2019 that the first patient had been dosed in the Phase I study with BI 765063 (OSE-172), a monoclonal antibody antagonist of SIRPα, that OSE licensed to BI. This triggered the second part of the €15m milestone payment from BI as expected (we only adjusted the milestone with 95% probability in our model). OSE is still eligible to receive up to a further €1.1bn in milestones plus royalties if certain milestones are met.
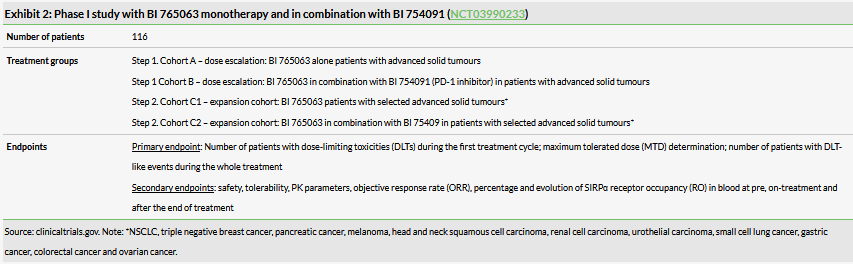
The full clinical trial design is now available (Exhibit 2). It is an open-label, dose-finding Phase I study of BI 765063 (OSE-172) as single agent and in combination with BI 754091 (anti-PD-1) to characterise safety, PK/PD and preliminary efficacy in patients with advanced solid tumours. OSE is responsible for running the trial but BI bears the costs, according to the collaboration and licensing agreement. BI will take over the development after Phase I.
In total 116 patients with advanced/metastatic tumours are expected to be enrolled. The study includes two steps and four cohorts, essentially two dose escalation cohorts and two expansion cohorts (BI 765063 standalone and BI 765063 in combination with BI’s own anti-PD-1). The dose-escalation cohorts are enrolling patients with advanced/metastatic primary or recurrent malignancies who failed or are not eligible to standard therapy. Then the expansion cohorts will be restricted to selected cancers (see Exhibit 2 notes for the list).
Combining BI 765063 with a PD-1 inhibitor is based on the complementary mechanisms of action of the two agents. That is, CD47/SIRPα inhibition should lead to phagocytosis of tumour cells by myeloid cells; this leads to tumour antigen uptake and presentation, which should activate the adaptive immune system, namely, T-cells killing tumour cells, which in turn is facilitated by PD-1/PD-L1 inhibition. OSE recently published an article describing the role of SIRPα in the induction and maintenance of immune tolerance and a poster presentation supporting the combination of a SIRPα monoclonal antibody and checkpoint inhibitor (anti-PD-L1 not anti-PD-1 as in the Phase I study). These pre-clinical studies add support to OSE’s strategy. As with many Phase I cancer trials, the indications are quite broad at the moment but will focus on the most promising cancers in further trials.
BiCKI bispecific platform: PD-1 + innovative targets
OSE presented its new BiCKI platform at the World Immunotherapy Congress in San Diego on 5 March 2019. This included some information about the bispecific/fusion antibody platform and included a lot of information on the first potential bispecific candidate, BiCKI IL-7. While we do not include any new assets in our valuation, we conducted a more detailed review of the platform and the opportunities.
Bispecifics and other types of antibodies can bind multiple targets. BiCKI is a bispecific/fusion antibody platform (Exhibit 3) that consists of:
A humanised high-affinity anti-PD-1 monoclonal antibody (blue Y in Exhibit 3). OSE has developed its own anti-PD-1 antibody that forms the ‘backbone’ of the bispecifics. So far there is limited information about this anti-PD-1 but OSE indicated it was chosen due to its good yield in terms of manufacturing and productivity, in particular in bispecific format. This is an IgG antibody in that it has a normal structure with heavy and light chains, where the Fab region binds to the PD-1 receptor on T-cells.
Engineered proteins attached to the Fc region (green circles in Exhibit 3). The silent Fc region of the antibody (base of the heavy chains) is the effector part of the molecule. It determines what effect the antibody has once it has bound to the antigen – usually an immune response. OSE plans to engineer different proteins to the Fc region, eg IL-7, through protein fusion to target novel receptors.
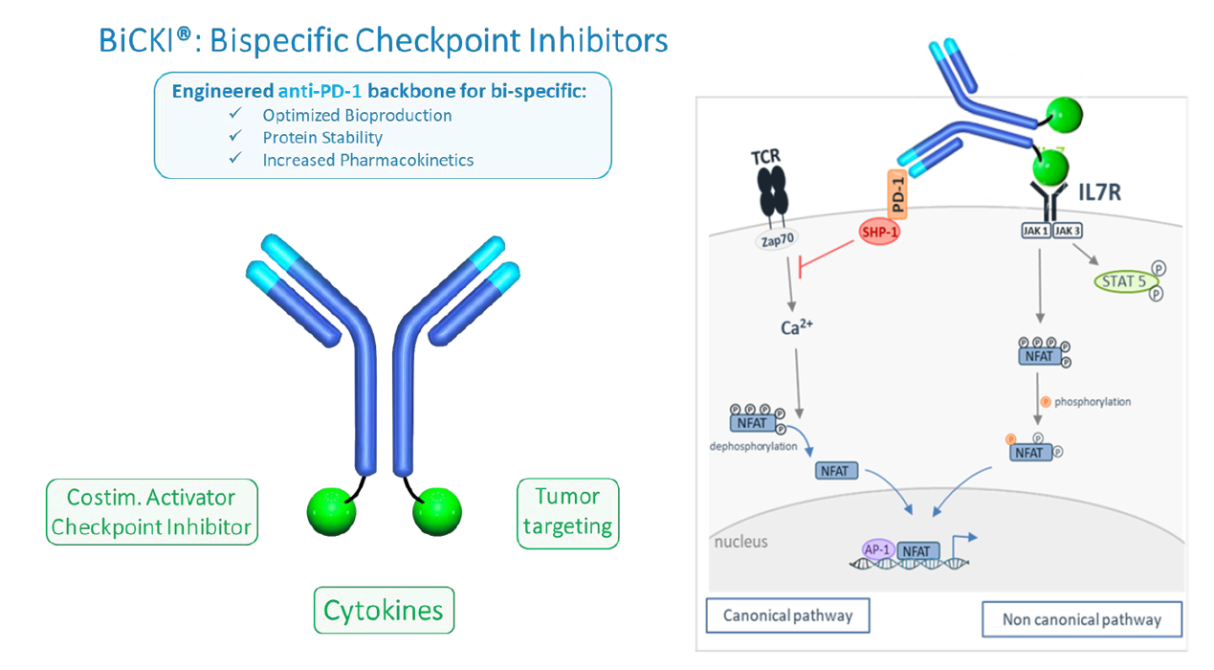
Exhibit 3: BiCKI platform (left) and BiCKI IL-7 mechanism of action on T-cells (right)
OSE hopes these anti-PD-1 bispecifics will help overcome the resistance (primary and secondary, depending on the second target) to anti-PD-1 drugs by selecting targets that are involved in different stages of the immunity cycle and so are complementary. In particular, OSE is looking for targets that would have synergistic potential.
OSE’s first candidate based on this platform is BiCKI IL-7 (Exhibit 3). OSE is already working with the IL-7 receptor (IL-7R) from the OSE-703 and OSE-127 programmes, so has experience in this area with a cytotoxic agonist asset. OSE suggested a few mechanisms of action for BiCKI IL-7 during the BiCKI presentation in March 2019, but perhaps the most important is that IL-7R sustains the life of exhausted T-cells. IL-7 is a cytokine that controls the proliferation, apoptosis and activation of CD4+ and CD8+ T-cells in humans. IL-7Rα expression is reduced in exhausted T-cells. Activating the IL-7Rα receptor with IL-7 should reduce T-cell exhaustion. The main advantage of the bispecific drug (over a combination of two drugs: anti-PD-1 with IL-7) is that BiCKI IL-7 synergistically increases TCR signalling and hence antigen-specific T cells responses.
OSE presented the first preclinical data with its BiCKI IL-7 technology at the International Cancer Immunotherapy Conference (CICON; 25–28 September, 2019) in Paris. The company showed that the bifunctional anti-PD1/IL-7 fusion protein potentiates effector function of exhausted T-cells and disarms the suppressive activity of regulatory T-cells.
Large biotech/pharma are getting in early on bispecifics
Many players are trying to tackle the resistance to checkpoint inhibitors issue through developing combination therapies and bispecifics to solve the same problem. Some existing projects in the anti-PD-1 bispecifics space are listed in Exhibit 4. The most advanced candidates are in Phase I. There are a few companies trying to use bispecifics to target multiple checkpoints, eg XmAb20717 targets both PD-1 and CTLA-4, with the aim of further reducing any T-cell inhibition by tumour cells and promoting T-cell activation.
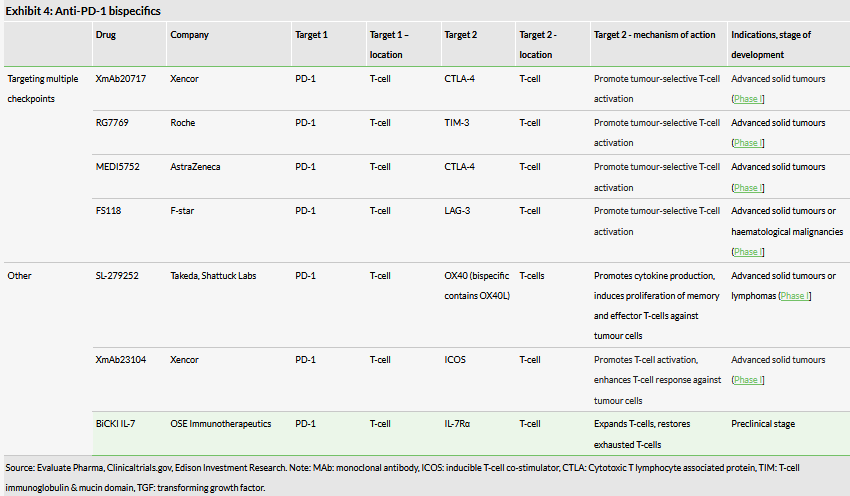
The bispecifics field has recently become a popular area. There are three bispecific antibodies (they do not target checkpoint receptors) that have been approved – Blincyto (blinatumomab, Amgen (NASDAQ:AMGN)), Hemlibra (emicizumab, Roche) and Removab (catumaxomab, Fresenius now withdrawn), but there are many more in clinical stages (57 individual bispecifics according to EvaluatePharma). They are being developed for both oncology and immunology indications.
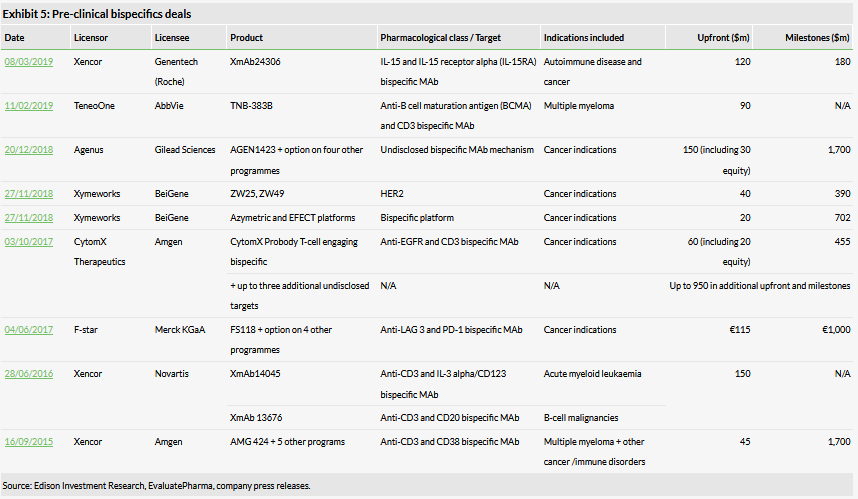
Most of the programmes are still very early stage therefore the existing deals are for bispecific platforms and pre-clinical programmes. There have been several large deals (Exhibit 5), which is promising for OSE. Most of the pre-clinical deals in Exhibit 5 include a collaboration based on the licensor’s bispecific platform plus rights for their existing pre-clinical bispecific programmes. These deals achieved between $45–150m in upfront payments and $180–1,700m in milestone payments (for platform plus existing programmes), depending on the number of candidates included in the deal. As there is an appetite for bispecifics platforms and bispecific candidates from pharma, OSE’s decision to invest in BiCKI seems well founded.
Financials
OSE reported a top line of €16.0m in H119, which was mostly a milestone from BI (the rest is R&D cost reimbursement to OSE). The milestone payment of €10m from Servier was booked as prepaid income (cash balanced with deferred income plus €2m in VAT received as OSE and Servier are both French companies). Total H119 operating expenses were €12.1m, of which R&D costs and overhead expenses amounted to €9.2m and €2.9m respectively, resulting in an operating profit of €3.9m. R&D costs are mainly associated with the Tedopi Phase III NSCLC study. Our full 2019 and 2020 estimated operating expenses were €19.6m and €19.7m, which we now revise to €22.7m and €22.8m.
As of end-H119, OSE had cash, cash equivalents and financial assets of €26.5m (includes ‘current financial assets’). The balance sheet also includes debt of €5.1m, which is mainly government loans. In September 2019, OSE announced it had received a €5.4m milestone payment from Bpifrance, the French public investment bank, in connection to the initiation of the Phase I trial with BI 765063 (€4.8m as a loan and €0.6m as income) as well. As part of a research consortium with the Léon Bérard cancer research centre, OSE also received a grant of €800k from the French National Research Agency, which will support preclinical research focused on validation of new targets linked to myeloid cells. Our model implies a comfortable cash reach to around end of 2020.
Valuation
We have raised our valuation of OSE to €198m or €13.2/share from €190m or €12.8/share previously. The increase is due to a better cash position and rolling our model forward, but is also a result of increased probability of success to a standard 10% for BI 765063 (OSE-172) as the Phase I trial has now been initiated (previously we used a slightly discounted probability of 7.5%). The milestone payment from Servier and BI had no effect as we have already included them in our model (now moved from respective projects to cash in Exhibit 6). We make no other changes to our assumptions, described in detail in our initiation report.
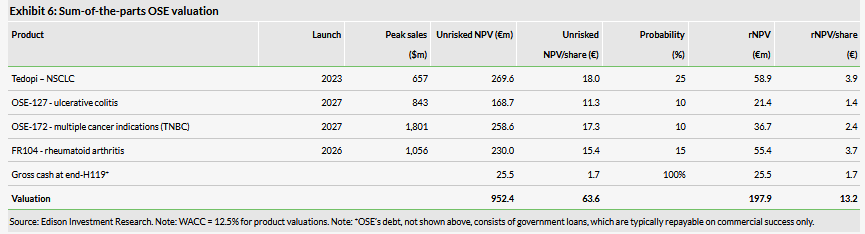
We include Tedopi NSCLC, FR104, OSE-127 and OSE-172 in our valuation. Although GERCOR has initiated the Phase II study with Tedopi in pancreatic cancer, we still do not include this indication in the valuation because its commercial strategy has not been confirmed – OSE will evaluate this based on the Phase II results. We do not include the new bispecific platform in the valuation because it is still in a pre-clinical stage.