Onxeo (CSE:ONXEO) is focused on the development of the next generation of DNA damage repair inhibitors from its novel oligonucleotide platON platform. The lead asset, AsiDNA, belongs to the same class of drugs as PARP inhibitors, but has a different mechanism of action. AsiDNA is in a Phase Ib trial in combination with chemotherapy in solid tumours; preliminary results are expected by end-2019, which is within the existing cash reach to Q320. To reflect the progress Onxeo has made with AsiDNA we have included the second indication for this asset in our valuation, but removed some of the legacy projects. Our updated valuation is €129m or €2.3/share.
Targeting already large and fast-growing market
Onxeo’s portfolio focuses on its novel platON platform, from which AsiDNA was the first product to enter clinical development. AsiDNA is the only oligonucleotide decoy agonist in development that disrupts and exhausts tumor DNA Damage Response mechanism. To date the only approved similar class drugs are four commercially successful PARP inhibitors (the first one was approved in December 2014). They all are indicated for cancers known to have a high degree of genomic instability, such as breast and ovarian cancers, which are also logical indications for AsiDNA. All four inhibitors are expected to generate total sales of US$1.6bn in 2019. DNA damage repair continues to attract significant attention and Onxeo’s acquisition of AsiDNA in early 2016 was well timed as it was before it became apparent how successful PARP inhibitors would become.
AsiDNA finishing Phase I development
Onxeo’s R&D plans include finishing the ongoing Phase Ib study with AsiDNA in various solid tumours in combination with chemotherapy (first data end-2019) and initiating a Phase Ib/II in combination with a PARP inhibitor in 2020. Then, depending on results and available funding, the company may continue with Phase II trials in these combinations. The rationale for combinations is the expected synergy with chemotherapy, but also AsiDNA’s unique ability to abrogate the resistance to PARP inhibitors seen in preclinical studies. Our base case scenario now includes two cancer indications (breast and ovarian) in various settings and assumes that Onxeo will be able to partner AsiDNA after Phase II.
Valuation: €129m or €2.3/share
We have revised our valuation to reflect Onxeo’s decision to focus solely on AsiDNA. We have included a second indication for AsiDNA, offset by a review of the Validive out-licensing deal, the removal of the residual rNPV of Beleodaq (the cash was received upfront after the royalty sale) and the removal of the small residual rNPV of Sitavig/Loramyc, two legacy specialty products.
Business description
Onxeo is focused on cancer indications, specialising in novel DNA damage response inhibitors. AsiDNA, a novel DNA break repair inhibitor from Onxeo’s platON platform, is in a Phase Ib trial with preliminary results expected in Q419. AsiDNA has a broad potential and can be combined with various anticancer treatments.
Investment summary
Company description: Focus on DNA damage repair
Onxeo is a French pure drug developer, which focuses on novel DNA damage repair inhibition technology. The technology came from the acquisition of DNA Therapeutics in March 2016, which brought the lead asset AsiDNA and a broad IP portfolio covering similar oligonucleotides that Onxeo organised into a platform technology called platON. Onxeo has one commercialised product, an HDAC inhibitor, belinostat, branded as Beleodaq in the US. AsiDNA is completing Phase Ib. Unlike similar class PARP inhibitors, AsiDNA is not dependent on specific gene mutations and therefore has broader application areas. In preclinical studies, Onxeo has shown that AsiDNA not only has synergistic potential in combinations with chemotherapeutic drugs that damage DNA (eg carboplatin), but also potentially has a unique feature to abrogate resistance to PARP inhibitors.
Valuation: Risk-adjusted NPV of €129m or €2.3/share
The main revision to our model is the AsiDNA potential. We now include two cancer indications (breast and ovarian cancer). AsiDNA’s potential is much broader in various other solid tumours and in combinations with different anticancer treatments including radiotherapy. For valuation purposes, we chose these two cancers, as they are of high priority in Onxeo’s near-term R&D plans. We assume Phase II development between 2020-2023, Phase III studies starting in 2023 and launch in 2026. We also assume that Onxeo will be able to partner AsiDNA after Phase II. We factor in R&D costs of €10m per cancer indication (the industry average for Phase II trials in oncology). This implies Onxeo will need to raise more funds, as the current cash reach is to Q320. We have used historical PARP inhibitor licensing deals as benchmark averages for our licensing model: total deal value of US$417m split into $40m upfront payment (roughly 10% of the total value) and the rest into R&D and commercial milestones (50:50) plus tiered 12–15% royalties.
Financials: Cash runway to Q320
Onxeo booked revenues of €6.1m in 2018, of which €2.3m were recurring Beleodaq sales. In June 2018, the company had effectively sold the Beleodaq royalties to SWK Holdings for US$7.5m upfront. Total operating costs were €9.7m in 2018 versus €28.7m in 2017. This fall was mainly due to lower R&D expenditure after the completion of the Phase III ReLive study in September 2017. For 2019 and 2020 we forecast total operating costs of €15.0m and €15.3m, respectively. Onxeo had a cash position of €6.3m at end-Q219. In June 2019, the company announced that it had renewed an equity financing line with Nice & Green, which based on the current share price level, would extend the cash reach to around Q320.
Sensitivities: Biotech risks apply
Onxeo is subject to the usual drug development risks, including clinical development delays or failures, regulatory risks, competitor successes, partnering setbacks and financing risks. The main sensitivities relate to the lead asset, AsiDNA. If Onxeo continues the development beyond the ongoing Phase Ib study it will require additional investment. The company aims to accumulate an attractive data package for the potential early out-licensing of AsiDNA (after Phase Ib). However, we believe that the value inflection that clinical trial readouts typically provide could be used to fund the Phase II studies, which would advance AsiDNA to late stage R&D and, presuming a successful clinical trial, would warrant much better licensing deal terms. Onxeo announced in October 2017 that the court’s decision in the litigation case was unfavourable and the company was ordered to pay €12m to SpeBio and other smaller charges to SpePharm/SpeBio for costs incurred to market Loramyc in Europe. Onxeo paid the required amount, but it owns a 50% stake in SpeBio, which is a joint venture with SpePharm, therefore the net impact is unclear at present, but Onxeo aims to claim back the proportional payment.
Outlook: Expanding DDR inhibitor R&D portfolio
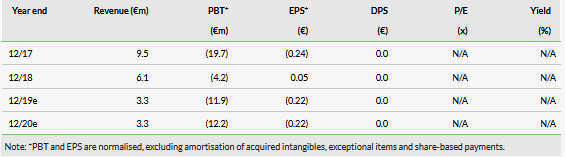
Onxeo’s portfolio focuses on its novel platON platform; in early 2018 AsiDNA was the first product from the platform to enter clinical development (Exhibit 1). The compounds on platON belong to the DDR inhibitor class, which is vital in DNA repair regulation. This area is currently attracting significant attention from both large pharma and biotech. To date, the only approved drugs are commercially successful PARP (poly(ADP (NASDAQ:ADP) ribose) polymerase) inhibitors.
Belinostat, an HDAC inhibitor, is out-licensed and commercialised as Beleodaq for the second line treatment of peripheral T-cell lymphoma. It is approved and marketed in the US following conditional approval and the partner bears the responsibility for any subsequent required studies. As Onxeo’s business model focuses on value creation from bringing preclinical assets through to clinical mid-stage, it effectively sold the royalty stream to SWK Holdings for $7.5m upfront in June 2018, raising non-dilutive funds for the development of AsiDNA.
platON: Novel technology platform with AsiDNA first to Phase I
Lead asset AsiDNA – acquisition background
Onxeo gained access to the AsiDNA asset and the IP, around which the platON platform was formed, via the acquisition of DNA Therapeutics in February 2016 (€1.7m upfront, €1m on Phase II initiation: in total up to €25m per approved indication can be paid out). The most advanced asset is AsiDNA (formerly known as DT01), a novel clinical-stage compound. It had already been tested in clinical trials by DNA Therapeutics and showed positive safety/tolerability results and preliminary anti-tumour activity in a Phase I trial with melanoma patients when administered locally. The deal terms appeared attractive and back-loaded for an asset that had Phase I data and could address major cancer indications in broad settings. This was probably due to the following, in our view:
the timing of the acquisition was very good, as the first approved PARP inhibitor, Lynparza (December 2014) had just finished its first year in the market generating sales of US$218m, therefore the R&D risk was somewhat lower, but the scale of the commercial success for this class of drugs was still not fully recognised (Lynparza is expected to generate US$1.1bn in sales in 2019 and the four approved (to date) PARP inhibitors are expected to generate total sales of US$1.6bn);
at the time of the acquisition AsiDNA was being explored only as an intratumourally administered anticancer drug, which limited the number of cancer indications it could target (intratumoural injection may still be explored by Onxeo).
With the commercial success of the PARP inhibitors, there is now significant interest in the area from various players and Onxeo’s acquisition timing means that AsiDNA is at the forefront of the next wave entering mid-to-late stage development.
Evolution of platON platform
Onxeo recognised that the potential of the technology behind AsiDNA could be increased substantially with systemic administration. Following the acquisition Onxeo completed a preclinical programme and repositioned the asset for systemic use. Onxeo’s main focus was on advancing AsiDNA to clinical trials, but based on the preclinical data the company also recognised the potential in other analogue compounds. In October 2017, this was formally introduced as a proprietary platform – platON.
The second platON asset that Onxeo has chosen to progress is OX401, which is now in the proof-of-concept preclinical phase (results expected by Q419). OX401 is also an oligonucleotide decoy agonist targeting PARP proteins and the STING (STimulator of INterferon Genes) pathway. STING is a relatively novel research target in immunoncology, which positions OX401 as a potential combination partner with other immunoncology agents, such as checkpoint inhibitors. In addition to systemic use, Onxeo continues to explore its oligonucleotide decoy technology’s potential for intratumoural administration.
AsiDNA –composition
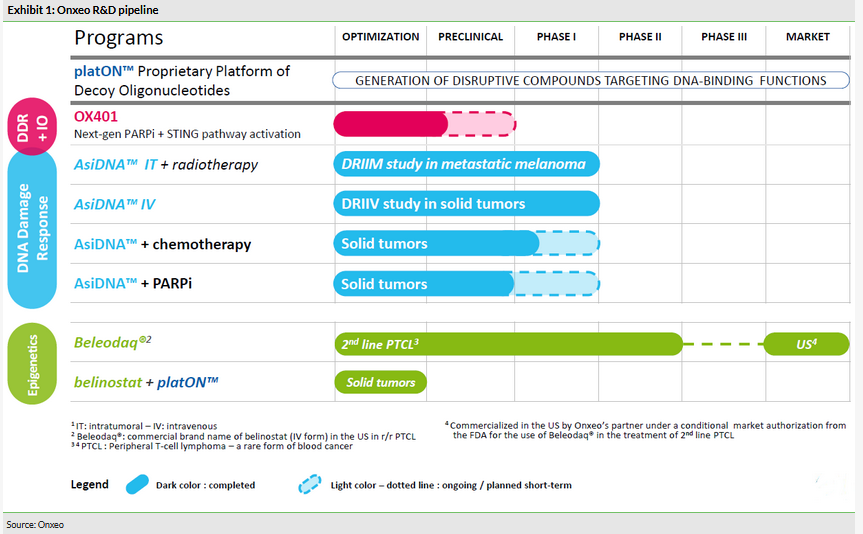
AsiDNA is a decoy oligonucleotide and is comprised of 64 nucleotides in two complementary strands (ie 32 nucleotides in each strand). In tumour cells AsiDNA acts as an agonist and interferes with the repair of tumour DNA by ‘distracting’ the tumour cell’s DNA repair mechanism. Decoy oligonucleotides are based on three components: double strand oligonucleotides, a linker (coupling agent) and a cellular uptake facilitator (Exhibit 2). Each of these compounds can be modified, resulting in different products, and the main mechanism of action is to act as a decoy and target the mechanisms of tumour DNA function regulation.
AsiDNA is now being tested in a Phase Ib trial in combination with classic chemotherapy agents (carboplatin + paclitaxel) in various solid tumours. Results are expected in Q419. Depending on available funding, near term development could include:
Phase Ib/II trial to assess synergy with chemotherapy in selected indications.
Phase Ib/II trial to assess the abrogation of resistance to PARP inhibitors, likely in maintenance treatment of advanced ovarian cancer.
DNA repair pathways and first breakthrough drugs
In the past five years four PARP inhibitors have been approved by the FDA, prompting a surge in interest in DNA repair inhibition.
olaparib (Lynparza, AstraZeneca); approved in December 2014, expected 2019 sales of US$1.1bn (EvaluatePharma), indicated for relapsed ovarian cancer in maintenance setting (BRCA mutated or non-mutated); and for second line treatment of metastatic breast cancer (BRCA mutated, HER2 negative).
rucaparib (Rubraca, Clovis Oncology); approved in December 2016, expected 2019 sales of US$149m, indicated for relapsed ovarian cancer in maintenance setting (BRCA mutated or non-mutated).
niraparib (Zejula, GSK/Tesaro); approved in March 2016, expected 2019 sales of US$310m, indicated for relapsed ovarian cancer in maintenance setting (BRCA mutated or non-mutated).
talazoparib (Talzenna, Pfizer (NYSE:PFE)); approved in October 2018, expected 2019 sales of US$44m, indicated for treatment of BRCA-mutated, HER2 negative metastatic breast cancer.
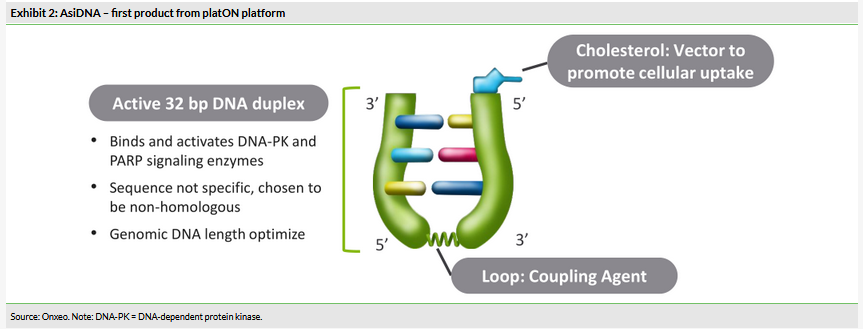
Regardless of the type of DNA lesion (endogenous-like replication errors or exogenous-like chemotherapy and radiation) cells initiate a highly coordinated cascade of events known as DNA damage response, which leads to the initiation of the damage repair mechanism specific to the type of the lesion (Exhibit 3). There are at least four main, partly overlapping, DNA repair pathways in mammals: base excision repair (BER), mismatch repair (MMR), nucleotide excision repair (NER) and double-strand break repair via two different pathways – homologous recombination (HR) and non-homologous end joining (NHEJ)1:
Single-strand breaks are repaired by BER. Other enzymes involved in BER are PARP1 and PARP2, which act as sensors and signal transducers1. PARP inhibition therefore affects this pathway specifically.
Double-strand breaks are the most serious lesions (one unrepaired double-strand break could trigger cell death). Primarily, these are repaired via two pathways, HR and NHEJ. The stage of cell cycle influences which mechanism is used. Among the important proteins involved in this pathway are BRCA1 and BRCA2.
NER pathway repairs a wide class of helix-distorting lesions that interfere with base pairing and obstruct transcription and normal replication.
MMR pathway repairs base mismatches that occur during cell DNA replication.
PARP inhibitors, the most advanced drugs in the DNA repair inhibition field, inhibit the BER pathway. This results in the accumulation of single-strand DNA breaks, eventually leading to double-strand breaks2. This process could cause cell death, but in healthy cells double-strand breaks are repaired via HR or NHEJ pathways. In a special case of mutated BRCA1/2 genes, the HR pathway is dysfunctional and these cells have been shown to be 100- to 1,000-fold more sensitive to PARP inhibition2. A biological defect such as mutated BRCA (dysfunctional HR), complemented by a drug leading to cell death, a PARP inhibitor in this case (blocks BER), is known as synthetic lethality.
BRCA1/2 mutations are found in around 15% of all cases of ovarian cancer and 5–10% of total breast cancer patients. The first three PARP inhibitors were approved for ovarian cancer. Initially they were explored in BRCA-mutated ovarian cancers, but efficacy has also been shown in BRCA non-mutated ovarian cancer as well. At a later stage, the label of the first PARP inhibitor, Lynparza, was extended to include metastatic breast cancer (BRCA mutated, HER2 negative) and the latest PARP inhibitor, talazoparib, which was approved in October 2018, is indicated for breast cancer only (BRCA-mutated, HER2 negative).
The rationale for using AsiDNA standalone and in combination
PARP inhibitors have shown promising efficacy and safety in clinical trials, but the main drawbacks are the necessity of a dysfunctional HR pathway and a rapid emergence of resistance2. First-in-class AsiDNA is based on signal-interfering DNA technology; if introduced into a cell it acts as a signal mimicking the damage of the cell’s own DNA. AsiDNA molecules are short double-strand DNA (32 nucleotides in each strand) that mimic double-strand breaks in the cell’s DNA and are recognised as ‘damaged DNA’ by repair and signalling proteins. Namely, AsiDNA hyper-activates PARP1 and DNA-PK leading to a cascade of repair proteins being recruited to ‘repair the damage’; as a result the actual damage of a cell’s DNA remains unrepaired. This action renders the HR and NHEJ pathways dysfunctional. Therefore, AsiDNA is clearly differentiated from PARP inhibitors, as it acts more upstream; it is not a specific enzyme inhibitor but activates PARP (ie ‘distracts’, when olaparib inhibits) among other repair proteins. This treatment approach has a broader action than PARP inhibitors, as it targets the entire DNA repair system by hijacking the DNA repair signalling.3
Since HR and NHEJ are responsible for repairing double-strand breaks, AsiDNA’s ability to disrupt these pathways was initially explored by the original inventors in combination with DNA-damaging therapies, such as radiotherapy and chemotherapy. Due to its independent mechanism of action there is also strong rationale to use AsiDNA in combination with PARP inhibitors to potentiate their effect in BRCA-mutated tumours. In addition, AsiDNA could potentially be used to sensitise BRCA non-mutated tumours to PARP inhibitors, which in turn would expand their use substantially.
Clinical development of AsiDNA
Phase I DRIIV-1: First trial with systemic AsiDNA administration
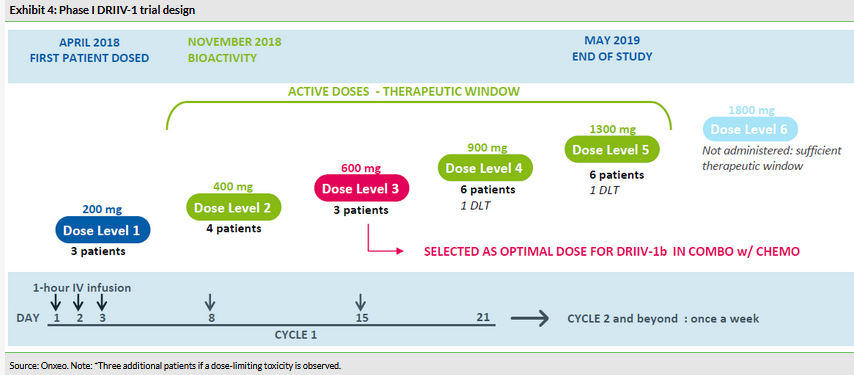
This was the first clinical trial Onxeo conducted after acquiring AsiDNA and is the most advanced dataset available at present. The open-label, dose escalation Phase I DNA Repair Inhibitor administered IntraVenously (DRIIV) recruited patients (n=22) with various advanced solid tumours, with the first patient treated in April 2018. All patients had metastatic cancers and were failing or progressing after one or more standard treatments with no further therapeutic options. The study aimed to assess:
The safety/tolerability profile, dose-limiting toxicities (DLTs) and the maximum tolerated dose (MTD), and the recommended dose for subsequent efficacy trials.
Onxeo also explored various biomarkers that might help to gauge the activity of AsiDNA and stratify patients in later trials.
The final results were announced in May 2019. The findings from a total of 22 patients who received five dose levels of AsiDNA ranging from 200mg to 1300mg include:
No serious drug-related events and no dose-limiting toxicity at doses 200, 400 and 600mg; first dose-limiting toxicity appeared at 900mg level (1/6).
Maximum tolerated dose not reached. There was no need to test the highest dose level (1800mg), as the therapeutic window between the optimal dose of 600mg and the highest tested dose of 1,300mg was considered sufficient.
Biomarker analysis showed that AsiDNA:
Increased activity of gH2AX and pHSP90 as early as the second level dose (400mg). γH2AX and pHSP90 are established biomarkers for the activation of DNA-PK, one of the major targets for AsiDNA.
Tumour proliferation biomarker Ki67 decreased.
The activity was consistent within the therapeutic window.
At the optimal dose level of 600mg, among the three patients included in the cohort, two patients with relapsed metastatic colorectal cancer were controlled with medical imaging, which showed no further disease progression after the second treatment cycle (the treatment with AsiDNA was continued for three months).
Our view
The rationale for this study was built on previous findings from the Phase I DRIIM, conducted by the inventor company. In that trial AsiDNA was administered intratumourally in melanoma patients. While the data released from the DRIIV-1 trial are still very early and no conclusions about efficacy can be made as yet, we find it reassuring that no serious drug-related side effects emerged with intravenous administration. The consistent pattern of increase in activity biomarkers also demonstrated that the drug reaches its target after intravenous administration. AsiDNA is a unique compound with no close comparators with respect to mechanism of action and therefore there was no visibility on the safety/tolerability profile via a systemic administration before DRIIV-1 results (safety data via local injection in the DRIIM trial was good).
Phase Ib DRIIV-1b (extension) study ongoing
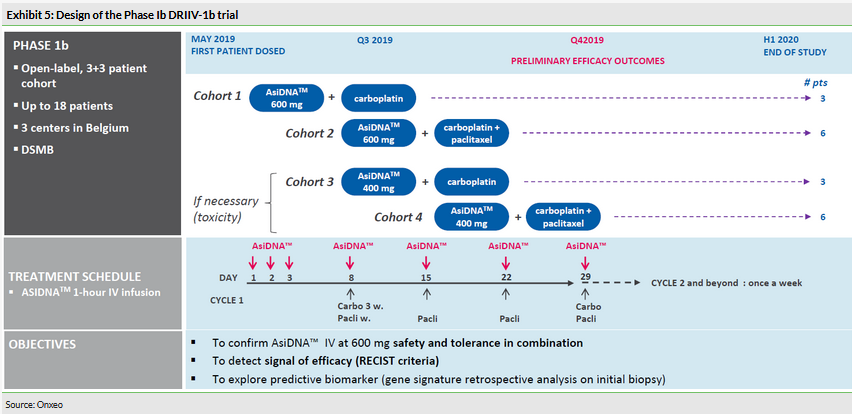
The next step in the development plan for AsiDNA is the extension of the Phase Ib (DRIIV-1b) study, which started enrolling patients in May 2019. This is the first study testing AsiDNA in combination with carboplatin plus paclitaxel, in up to 18 patients (9 + 9 if dose-limiting toxicity is reached; Exhibit 5) with solid tumours eligible for such treatments (such as lung, breast, ovarian or head and neck cancers). The study will evaluate the efficacy of the combination treatment using RECIST criteria (response evaluation criteria in solid tumours) and the initial results are expected in Q419.
Future development options for AsiDNA and partnering strategy
As discussed above, the AsiDNA’s mechanism of action allows it to be positioned in various combinations with other anticancer therapies. Therefore, near-term R&D plans include studies with AsiDNA in combination with classic chemotherapy drugs and novel PARP inhibitors (Exhibit 6). We note that only the Phase Ib DRIIV-1b study is ongoing; other trials are at the planning stage and the results from the DRIIV-1b trial will help to design other studies. The final decision on which indications to prioritise will be made in the near future, but ovarian and breast cancer seem to be most likely targets at present. In this regard, we find the Phase Ib/II study with AsiDNA in combination with PARP inhibitors of particular interest. It will explore AsiDNA’s potential to abrogate tumour resistance to PARP inhibitors. There is a strong rationale for such a combination and the proof-of-concept was demonstrated in the animal studies (discussed below). To date, the four approved PARP inhibitors have been approved for the treatment of ovarian or breast cancer. While commercially successful drugs, they still suffer from the rapid development of resistance, so AsiDNA’s benign safety profile could be a good partner drug because of its ability to abrogate resistance to the drug.

When it comes to late-stage development, Onxeo’s business strategy implies it will seek to partner or out-license the development of AsiDNA. Central to Onxeo’s investment case is its ability to secure a timely out-licensing agreement. The company would consider out-licensing soon after the ongoing Phase Ib trial, which would allow the company to focus on earlier stage products in the platON platform. To be prepared for that outcome Onxeo is accumulating as broad data package as possible. However, as Onxeo employs an early- to mid-stage development biotech business model, the data readouts, if positive, should create value inflection points for the share price, which would allow it to raise the necessary funds for the Phase II development. This option would lead to better deal terms and better returns to investors willing to take on the Phase II development risk.
Our base case scenario
In our model we assume that Onxeo will be able to partner AsiDNA after Phase II and the partner will cover all development and marketing costs from this point. We include two cancer indications (breast and ovarian cancer; detailed below) in our Onxeo rNPV valuation model. We assume the two Phase II studies are initiated in 2020, Phase III studies in 2023 and launch in 2026. The R&D cost for each of the two Phase II trials is €10m (industry average Phase II trials in oncology). This implies Onxeo will need to raise more funds, as the current cash reach is to Q320 (see Financials). Our partnering assumptions include a fairly typical deal structure, including an upfront payment, development and sales-related milestones, in addition to royalties on global sales. We assume that AsiDNA will be out-licensed after the Phase II studies, ie in 2022. We have used historical PARP inhibitor licensing deals as benchmarks for our valuation (Exhibit 17). In our model we include the total deal value of US$417m (average of the values of the three deals). This is split into an upfront payment of US$40m (roughly 10% of the total value) with the rest split into R&D and commercial milestones. These values are equally split between the two indications. We use tiered 12–15% royalty rates. We summarise our other valuation assumptions later in the report.
Phase I DRIIM results (AsiDNA via local administration)
DNA Therapeutics, the original developer, tested AsiDNA (DT01 at that time) in a clinical trial with skin melanoma patients. In the Phase I DRIIM study the drug was injected intratumourally or peritumourally in conjunction with radiation therapy. DRIIM was an open-label, non-randomised, multicentre, dose escalation study. In total, 23 patients received a full course of treatment and were evaluated for safety and pharmacokinetics, while 21 patients with a total of 76 skin melanoma lesions were evaluated for initial efficacy. Key headline results were presented at ASCO in May 2015 and published later4:
AsiDNA was well tolerated and did not induce additional toxicity when combined with radiotherapy. The maximum tolerated dose was not reached.
AsiDNA did not cause innate immune response, which would imply that the drug is less likely to be neutralised by the immune system or cause unwanted significant local inflammation.
In the 21 patients that were evaluated for efficacy, a total of 76 tumour lesions were treated, of which 41 lesions were injected with AsiDNA.
The objective response rate of all lesions was 59%, complete response was 30% and partial response was 29%. For comparison, similar radiation therapy schemes were reported to have a complete response rate of 9%4.
The overall response rate of the 41 lesions injected with AsiDNA was 68% whereas in the 35 non-injected lesions it was 49% (P=0.103). This lack of significant difference could in part be explained by systemic exposure to AsiDNA after it was absorbed from the local injection site and an abscopal effect through immunogenicity, ie the immune system was trained to attack both the injected tumours and the non-injected.
These results showed that AsiDNA was well-tolerated and had an effect on lesions when injected locally and that a systemic delivery is also possible. As discussed, we believe that this potential to reposition AsiDNA for systemic delivery was the primary driver for Onxeo’s acquisition of DNA Therapeutics in March 2016.
Preclinical data highlights
First proof-of-concept of AsiDNA systemic administration
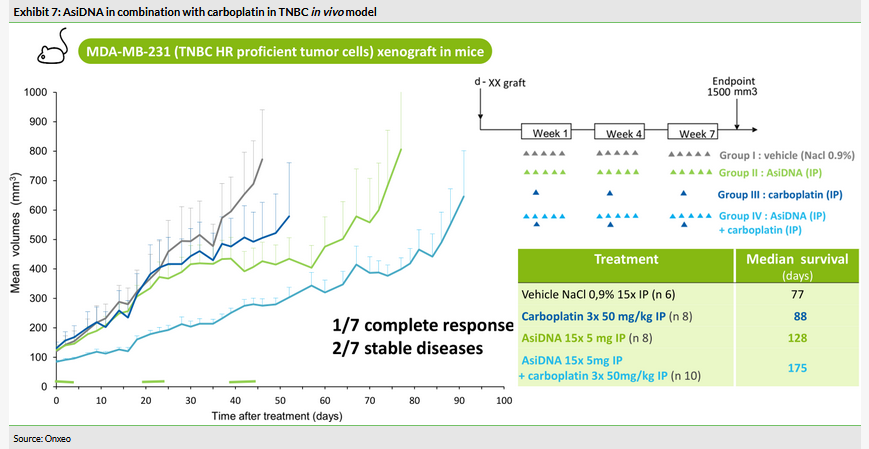
After Onxeo acquired AsiDNA it completed the required additional preclinical development and the first preclinical proof-of-concept data demonstrating the potential for intravenous administration were released in July 2017. AsiDNA standalone significantly decreased tumour growth in triple negative breast cancer (TNBC) model and improved survival, while a combination with the classic chemotherapy agent carboplatin significantly reduced the growth further and outperformed all other arms (Exhibit 7).
To read the entire report Please click on the pdf File Below..