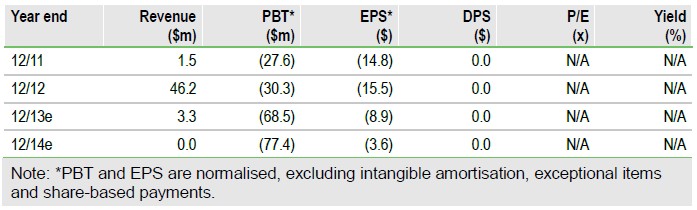
Down but not out
Rigosertib did not meet its primary endpoint of overall survival (OS) in the Phase III ONTIME trial in higher-risk myelodysplastic syndromes (MDS), but showed a statistically significant OS improvement in the subgroup of patients who had progressed or failed on hypomethylating agents (HMAs). The news is a setback, but there remains a path forward for rigosertib in a substantial subpopulation of higher-risk MDS in our opinion. We have reduced our Onconova Th, (ONTX) valuation to $303m or $14.1/basic share by lowering rigosertib sales estimates and the probability of success in higher-risk MDS.
ONTIME missed its primary endpoint…
The ONTIME Phase III trial compared rigosertib IV to best supportive care (BSC) in higher-risk MDS patients who had progressed, failed or relapsed after previous HMA therapy. Although there was a numerical advantage favouring rigosertib (median OS was 8.2 months vs 5.8 months for control) and a hazard ratio (HR) of 0.86, this did not achieve the p<0.05 level of statistical significance (p=0.27). Rigosertib performed as expected (the Phase III design assumed a median OS of c 35 weeks, or 8.16 months), but the BSC arm exceeded expectations (assumed median OS of 17-22 weeks, or 3.96-5.13 months).
…but showed efficacy in a post hoc subgroup
However, a post hoc analysis of efficacy in subgroups found rigosertib to be efficacious in patients who progressed or failed previous treatment with HMAs. The median OS was 8.5 vs 4.7 months, HR of 0.67 and p-value of 0.022. The subgroup was not predefined, but nevertheless represents a substantial proportion (184 of 299, or 61.5%) of patients in the study. It is also clinically distinct (all patients are HMA non-responders) and the most challenging to treat (HMA failures have the poorest prognosis), and therefore has the greatest clinical need. For these reasons, we consider rigosertib could be further developed for this patient group, pending a dialogue with the FDA in the near future.
Valuation: Reduced to $303m
We have reduced our valuation to $303m by lowering the rigosertib sales estimates (peak sales $305m, down from previously $625m in higher-risk MDS) and the assumed probability of clinical success rate to 50% (from 65%). Given the share price reaction to the news, this suggests there is still upside to the shares if development of rigosertib for higher-risk, HMA non-responder MDS patients can be continued.
To Read the Entire Report Please Click on the pdf File Below